For US Healthcare Professionals
I am a:
For US Healthcare Professionals
Full Prescribing InformationJoint improvement proven to last at 2 years (ACR20)1-5*†‡§II¶
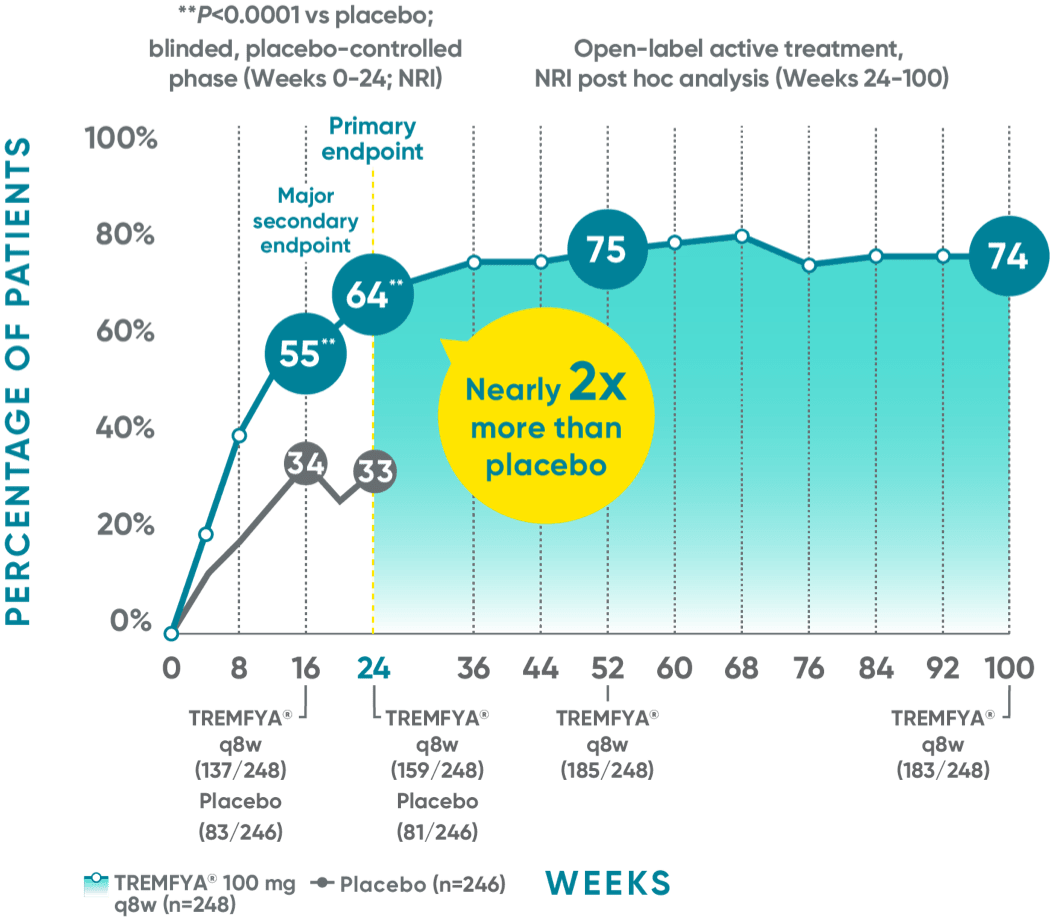
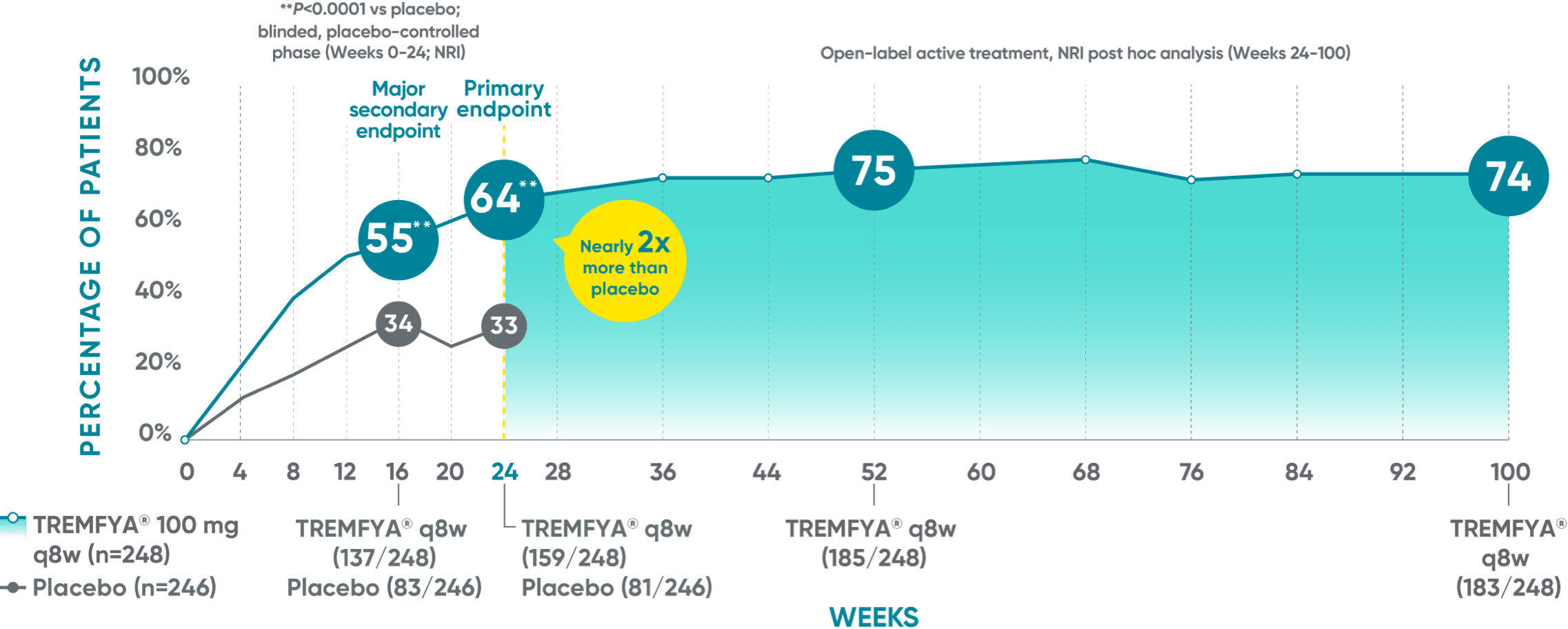
DISCOVER 2 (ACR20): Continued improvement in joint symptoms# at 2 years*†


In DISCOVER 1:
Week 24 (major secondary endpoint): 52% of patients receiving TREMFYA® q8w (66/127) achieved an ACR20 response vs 22% of patients receiving placebo (28/126) (P<0.0001)1,2,6‡§
Week 24 (primary endpoint): 52% of patients receiving TREMFYA® q8w (66/127) achieved an ACR20 response vs 22% of patients receiving placebo (28/126) (P<0.0001)1,2,6‡§
Week 16: 52% of patients receiving TREMFYA® q8w (66/127) achieved an ACR20 response vs 25% of patients receiving placebo (32/126) (P<0.0001)1,2‡§
Week 52 (nonresponder imputation [NRI] post hoc analysis): 60% of patients receiving TREMFYA® q8w (76/127) achieved an ACR20 response1,7‡§ǁ††
ACR20 response in DISCOVER 1 and DISCOVER 2 at Week 16 was not part of the sequential testing procedure but was prespecified to be tested upon achieving statistical significance for ACR20 at Week 24.
*Year 2 represents Week 100.
†The same patients may not have responded at each time point.
‡Through Week 24, patients were considered to be nonresponders after meeting treatment failure criteria: discontinued study agent for any reason, terminated study participation for any reason, initiated or increased the dose of disease-modifying antirheumatic drugs (DMARDs) or oral corticosteroids over baseline for PsA, or initiated protocol-prohibited medications/therapies for PsA. After Week 24, treatment failure rules were not applied.
§Patients with missing data were considered nonresponders.
||After Week 24, patients and doctors knew that all patients were on TREMFYA® (open label with a blinded dosing interval), which may have affected the results.
¶The DISCOVER 2 prespecified as-observed analysis from Weeks 24 to 100 is not shown.
#Based on ACR response rates.
††The DISCOVER 1 prespecified as-observed analysis from Weeks 24 to 52 is not shown.
References: 1. Data on file. Janssen Biotech, Inc. 2. TREMFYA® (guselkumab) [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc. 3. Mease PJ, Rahman P, Gottlieb AB, et al. Guselkumab in biologic-naïve patients with active psoriatic arthritis (DISCOVER-2): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395(10230):1126-1136. 4. McInnes IB, Rahman P, Gottlieb AB, et al. Efficacy and safety of guselkumab, an interleukin-23p19-specific monoclonal antibody, through one year in biologic-naïve patients with psoriatic arthritis. Arthritis Rheumatol. 2021;73(4):604-616. 5. McInnes IB, Rahman P, Gottlieb AB, et al. Efficacy and safety of guselkumab, a monoclonal antibody specific to the p-19 subunit of interleukin-23, through two years: results from a phase III, randomized, double-blind, placebo-controlled study conducted in biologic-naïve patients with active psoriatic arthritis. Arthritis Rheumatol. 2022;74(3):475-485. 6. Deodhar A, Helliwell PS, Boehncke W-H, et al. Guselkumab in patients with active psoriatic arthritis who were biologic-naïve or had previously received TNFα inhibitor treatment (DISCOVER 1): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395(10230):1115-1125. 7. Ritchlin CT, Helliwell PS, Boehncke W-H, et al. Guselkumab, an inhibitor of the IL-23p19 subunit, provides sustained improvement in signs and symptoms of active psoriatic arthritis: 1 year results of a phase III randomised study of patients who were biologic-naïve or TNFα inhibitor-experienced. RMD Open. 2021;7(1):e001457.
Joint improvement proven to last at 2 years (ACR50)1-5*†‡§II¶
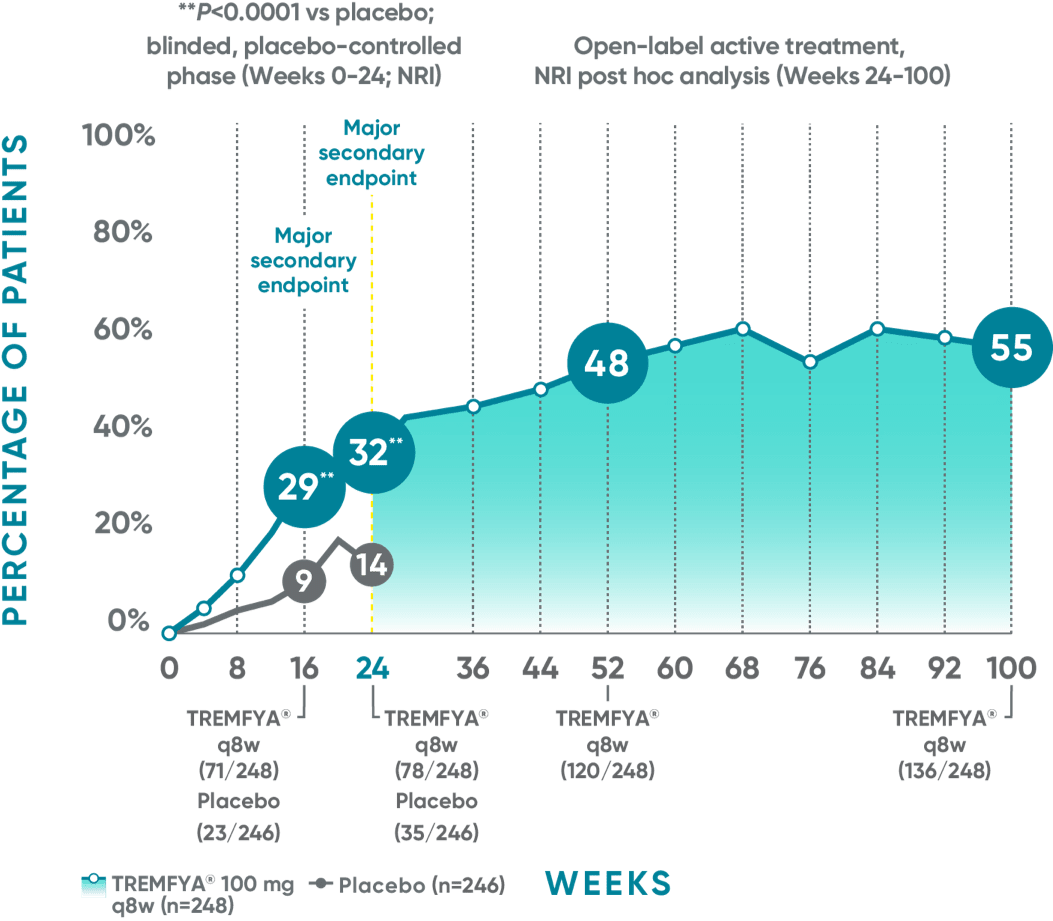
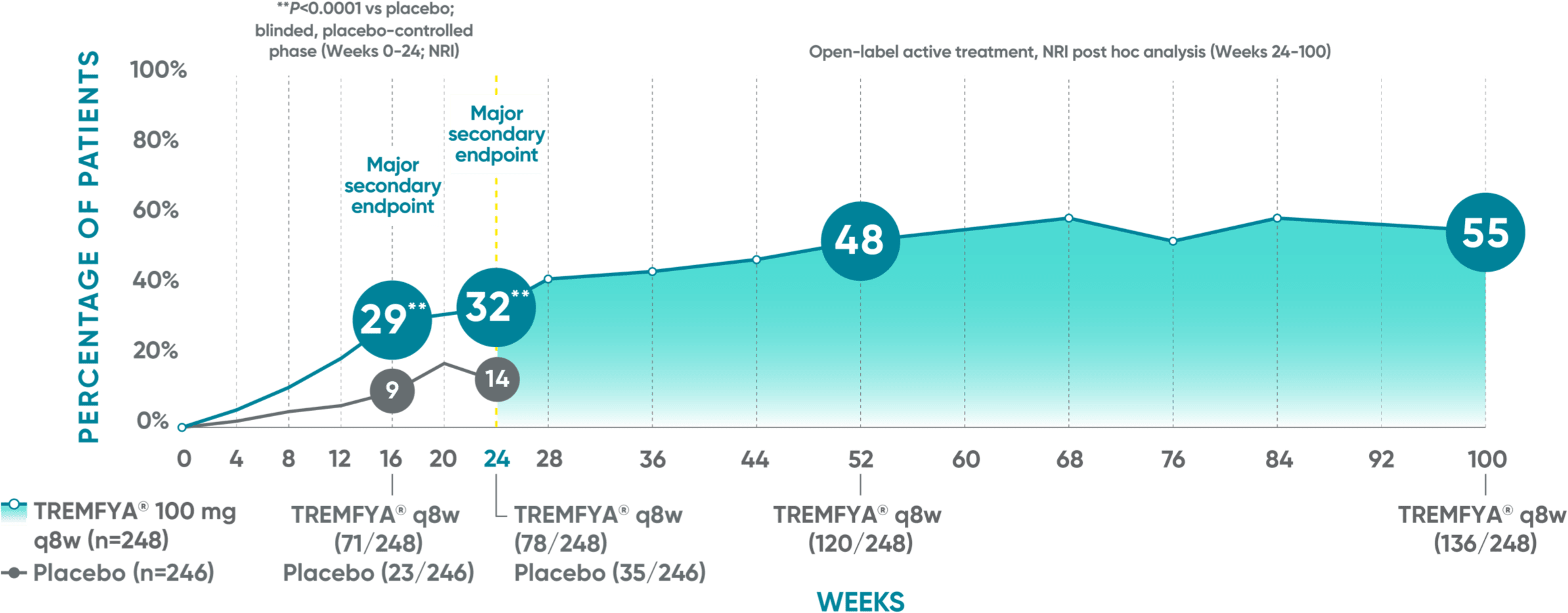
DISCOVER 2 (ACR50): Continued improvement in joint symptoms# at 2 years*†


In DISCOVER 1:
Week 24 (major secondary endpoint): 30% of patients receiving TREMFYA® q8w (38/127) achieved an ACR50 response compared with 9% of patients receiving placebo (11/126) (P<0.0001)1,2,6‡§
Week 16: 23% of patients receiving TREMFYA® q8w (29/127) achieved an ACR50 response vs 13% of patients receiving placebo (16/126) (P=0.036)1,2‡§
Week 52 (NRI post hoc analysis): 39% of patients receiving TREMFYA® q8w (49/127) achieved an ACR50 response1,7‡§ǁ††
ACR50 responses in DISCOVER 1 and DISCOVER 2 at Weeks 16 and 24 were not part of the sequential testing procedure but were prespecified to be tested upon achieving statistical significance for ACR20 response at Week 24.
*The same patients may not have responded at each time point.
†Year 2 represents Week 100.
‡Through Week 24, patients were considered to be nonresponders after meeting treatment failure criteria: discontinued study agent for any reason, terminated study participation for any reason, initiated or increased the dose of disease-modifying antirheumatic drugs (DMARDs) or oral corticosteroids over baseline for PsA, or initiated protocol-prohibited medications/therapies for PsA. After Week 24, treatment failure rules were not applied.
§Patients with missing data were considered nonresponders.
||After Week 24, patients and doctors knew that all patients were on TREMFYA® (open label with a blinded dosing interval), which may have affected the results.
¶The DISCOVER 2 prespecified as-observed analysis from Weeks 24 to 100 is not shown.
#Based on ACR response rates.
††The DISCOVER 1 prespecified as-observed analysis from Weeks 24 to 52 is not shown.
NRI=nonresponder imputation.
References: 1. Data on file. Janssen Biotech, Inc. 2. TREMFYA® (guselkumab) [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc. 3. Mease PJ, Rahman P, Gottlieb AB, et al. Guselkumab in biologic-naïve patients with active psoriatic arthritis (DISCOVER-2): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395(10230):1126-1136. 4. McInnes IB, Rahman P, Gottlieb AB, et al. Efficacy and safety of guselkumab, an interleukin-23p19-specific monoclonal antibody, through one year in biologic-naïve patients with psoriatic arthritis. Arthritis Rheumatol. 2021;73(4):604-616. 5. McInnes IB, Rahman P, Gottlieb AB, et al. Efficacy and safety of guselkumab, a monoclonal antibody specific to the p-19 subunit of interleukin-23, through two years: results from a phase III, randomized, double-blind, placebo-controlled study conducted in biologic-naïve patients with active psoriatic arthritis. Arthritis Rheumatol. 2022;74(3):475-485. 6. Deodhar A, Helliwell PS, Boehncke W-H, et al. Guselkumab in patients with active psoriatic arthritis who were biologic-naïve or had previously received TNFα inhibitor treatment (DISCOVER 1): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395(10230):1115-1125. 7. Ritchlin CT, Helliwell PS, Boehncke W-H, et al. Guselkumab, an inhibitor of the IL-23p19 subunit, provides sustained improvement in signs and symptoms of active psoriatic arthritis: 1 year results of a phase III randomised study of patients who were biologic-naïve or TNFα inhibitor-experienced. RMD Open. 2021;7(1):e001457.
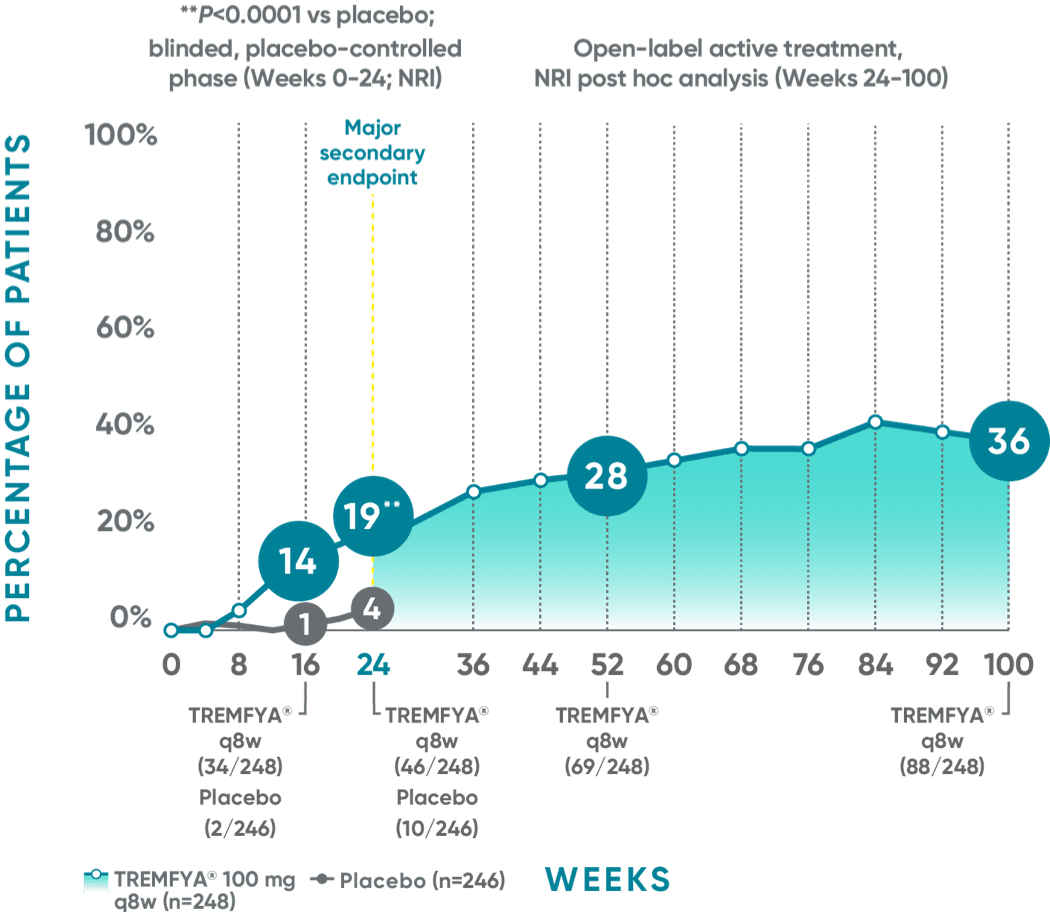
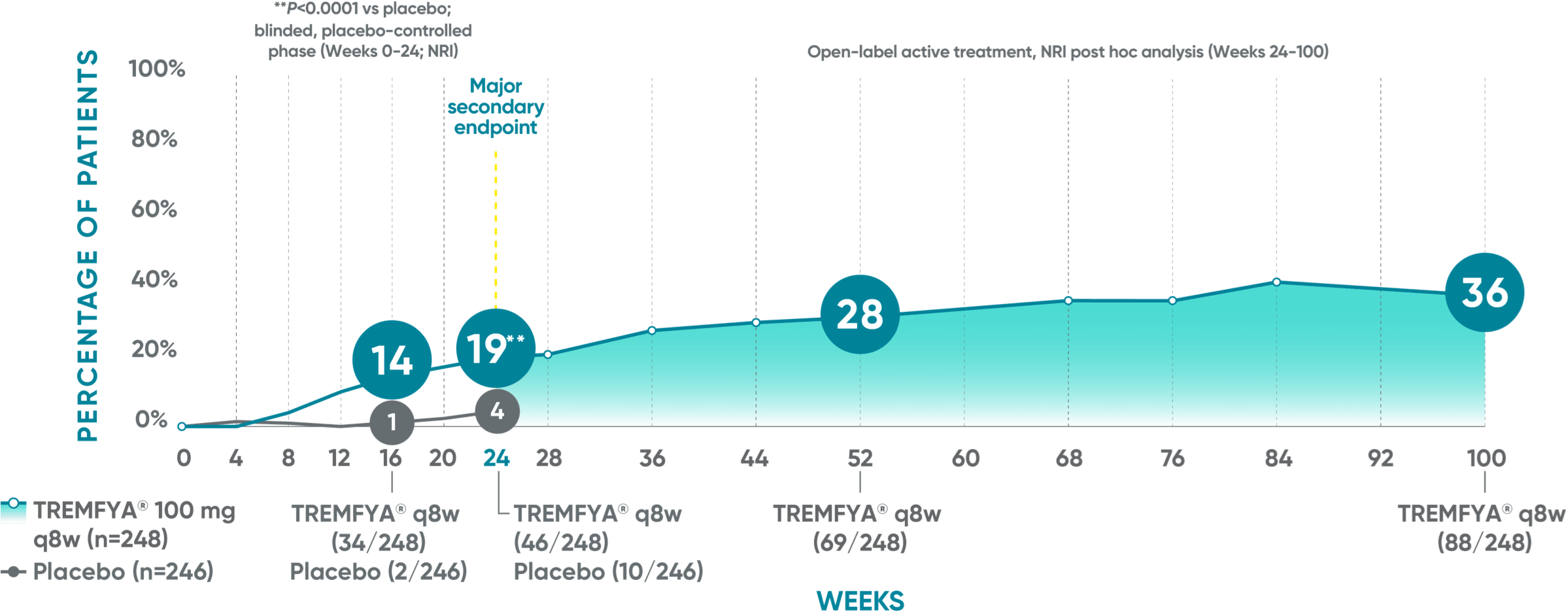
Joint improvement proven to last at 2 years (ACR70)1-5*†‡§II¶
DISCOVER 2 (ACR70): Continued improvement in joint symptoms# at 2 years*†


In DISCOVER 1:
Week 24 (major secondary endpoint): 12% of patients receiving TREMFYA® q8w (15/127) achieved an ACR70 response vs 6% of patients receiving placebo (7/126) (P=0.069)1,2,6‡§
Week 16: 8% of patients receiving TREMFYA® q8w (10/127) achieved an ACR70 response vs 6% of patients receiving placebo (7/126)1,2‡§
Week 52 (NRI post hoc analysis): 26% of patients receiving TREMFYA® q8w (33/127) achieved an ACR70 response1,7‡§ǁ††
ACR70 responses in DISCOVER 1 and DISCOVER 2 at Week 24 were not part of the sequential testing procedure but were prespecified to be tested upon achieving statistical significance for ACR20 response at Week 24.
ACR70 response in DISCOVER 1 and DISCOVER 2 at Week 16 was not controlled for multiplicity. Therefore, statistical significance has not been established.
*The same patients may not have responded at each time point.
†Year 2 represents Week 100.
‡Through Week 24, patients were considered to be nonresponders after meeting treatment failure criteria: discontinued study agent for any reason, terminated study participation for any reason, initiated or increased the dose of disease-modifying antirheumatic drugs (DMARDs) or oral corticosteroids over baseline for PsA, or initiated protocol-prohibited medications/therapies for PsA. After Week 24, treatment failure rules were not applied.
§Patients with missing data were considered nonresponders.
||After Week 24, patients and doctors knew that all patients were on TREMFYA® (open label with a blinded dosing interval), which may have affected the results.
¶The DISCOVER 2 prespecified as-observed analysis from Weeks 24 to 100 is not shown.
#Based on ACR response rates.
††The DISCOVER 1 prespecified as-observed analysis from Weeks 24 to 52 is not shown.
NRI=nonresponder imputation.
References: 1. Data on file. Janssen Biotech, Inc. 2. TREMFYA® (guselkumab) [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc. 3. Mease PJ, Rahman P, Gottlieb AB, et al. Guselkumab in biologic-naïve patients with active psoriatic arthritis (DISCOVER-2): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395(10230):1126-1136. 4. McInnes IB, Rahman P, Gottlieb AB, et al. Efficacy and safety of guselkumab, an interleukin-23p19-specific monoclonal antibody, through one year in biologic-naïve patients with psoriatic arthritis. Arthritis Rheumatol. 2021;73(4):604-616. 5. McInnes IB, Rahman P, Gottlieb AB, et al. Efficacy and safety of guselkumab, a monoclonal antibody specific to the p-19 subunit of interleukin-23, through two years: results from a phase III, randomized, double-blind, placebo-controlled study conducted in biologic-naïve patients with active psoriatic arthritis. Arthritis Rheumatol. 2022;74(3):475-485. 6. Deodhar A, Helliwell PS, Boehncke W-H, et al. Guselkumab in patients with active psoriatic arthritis who were biologic-naïve or had previously received TNFα inhibitor treatment (DISCOVER 1): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395(10230):1115-1125. 7. Ritchlin CT, Helliwell PS, Boehncke W-H, et al. Guselkumab, an inhibitor of the IL-23p19 subunit, provides sustained improvement in signs and symptoms of active psoriatic arthritis: 1 year results of a phase III randomised study of patients who were biologic-naïve or TNFα inhibitor-experienced. RMD Open. 2021;7(1):e001457.
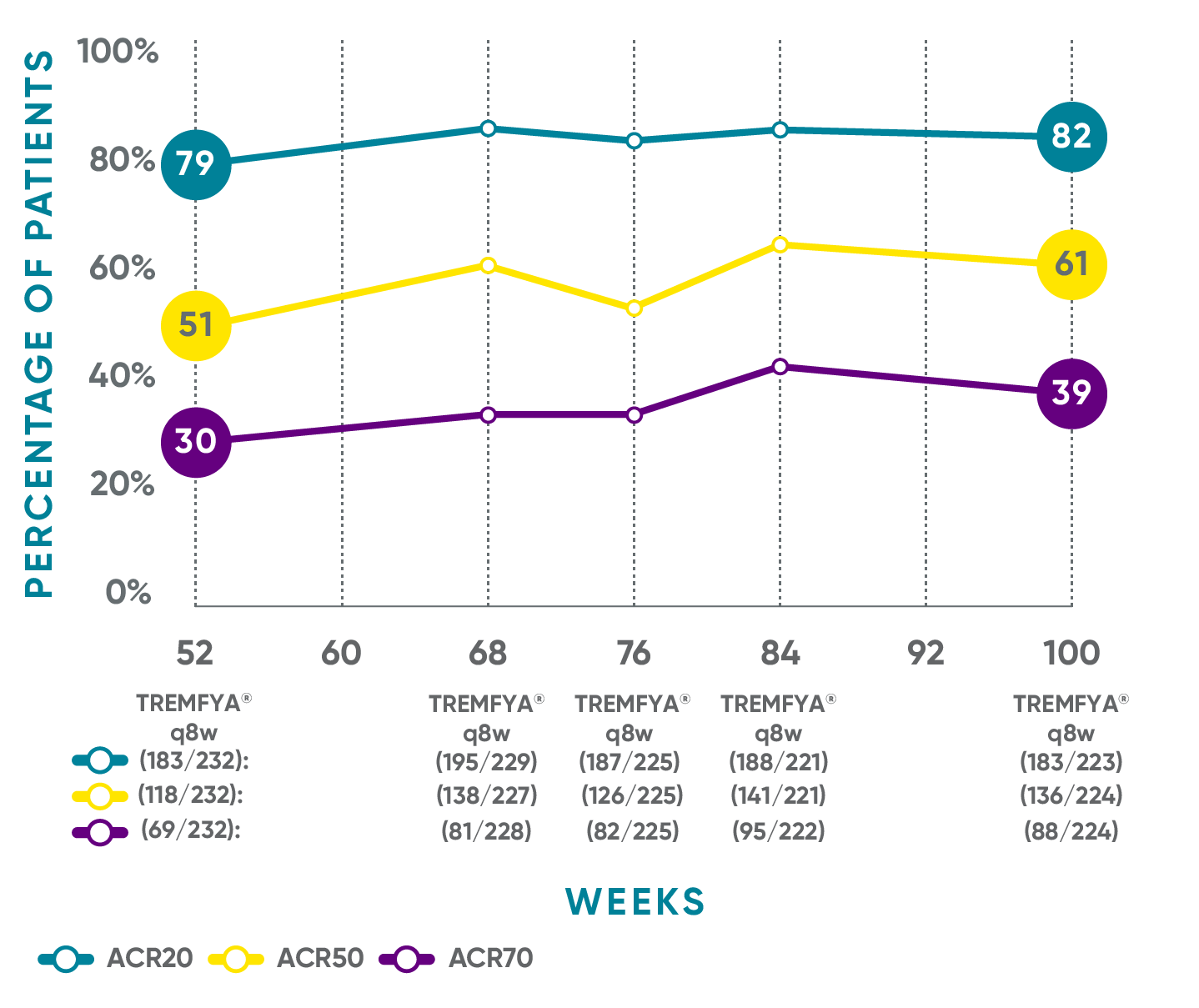
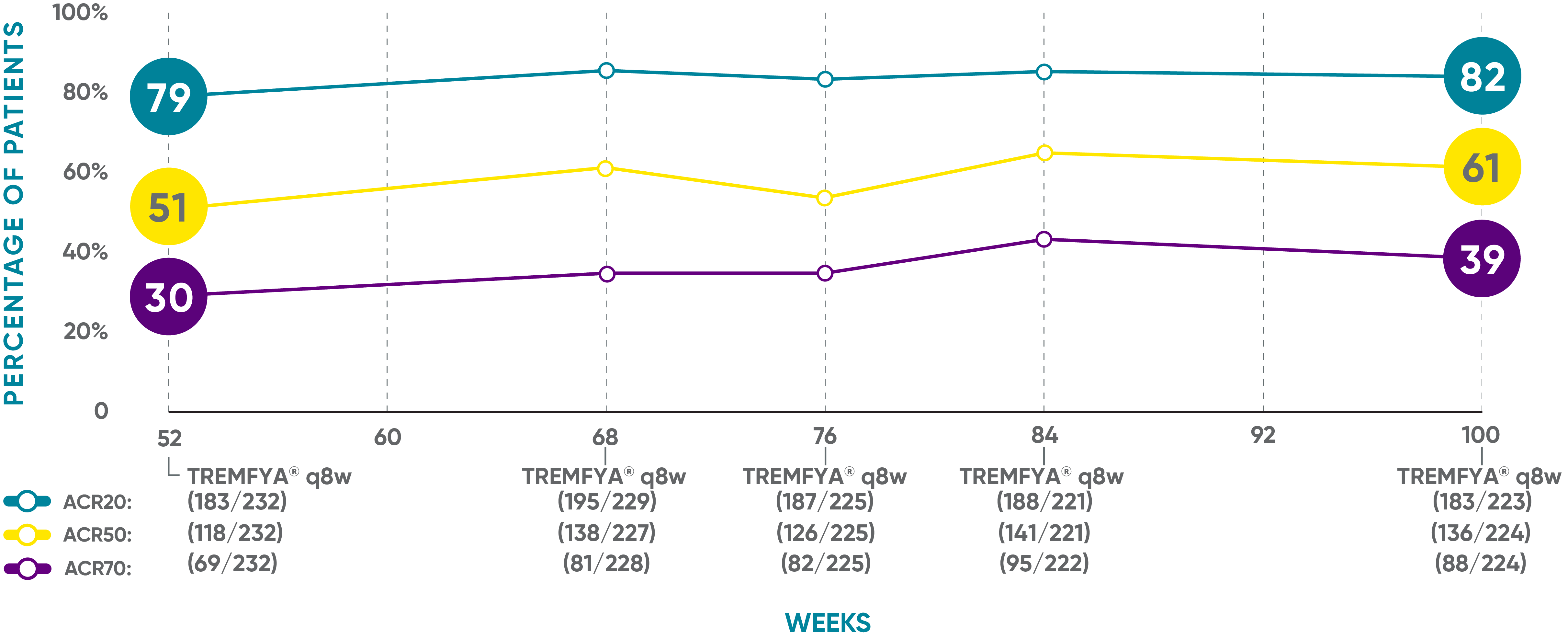
Sustained joint improvement* at 2 years1,2†‡§II¶#**
DISCOVER 2: ACR20, ACR50, and ACR70 response rates from Week 52 through Week 100 (as-observed analysis)
Open-label active treatment (prespecified as-observed analysis)


In DISCOVER 1 (Week 52)3:
ACR responses (as observed)*†‡ǁ¶**
ACR20 response
68%
TREMFYA®
(76/112)
ACR50 response
43%
TREMFYA®
(49/113)
ACR70 response
29%
TREMFYA®
(33/114)
*Patients who lose response or are unable to tolerate treatment are likely to discontinue treatment, which may increase the response rate in an as-observed analysis.
†The same patients may not have responded at each time point.
‡After Week 24, patients and doctors knew that all patients were on TREMFYA® (open label with a blinded dosing interval), which may have affected the results.
§Year 2 represents Week 100.
||Available data at each visit were used; missing data were not included in the analysis.
¶The prespecified as-observed analysis from Weeks 24 to 52 is not shown, but the prespecified as-observed analysis at Week 52 is shown.
#Includes all randomized subjects still on study treatment at Week 52.
**Includes all randomized subjects still on study treatment at Week 24.
References: 1. TREMFYA® (guselkumab) [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc. 2. Data on file. Janssen Biotech, Inc. 3. Ritchlin CT, Helliwell PS, Boehncke W-H, et al. Guselkumab, an inhibitor of the IL23p19 subunit, provides sustained improvement in signs and symptoms of active psoriatic arthritis: 1 year results of a phase III randomized study of patients who were biologic-naive or TNFα inhibitor-experienced. RMD Open. 2021;7(1):e001457.
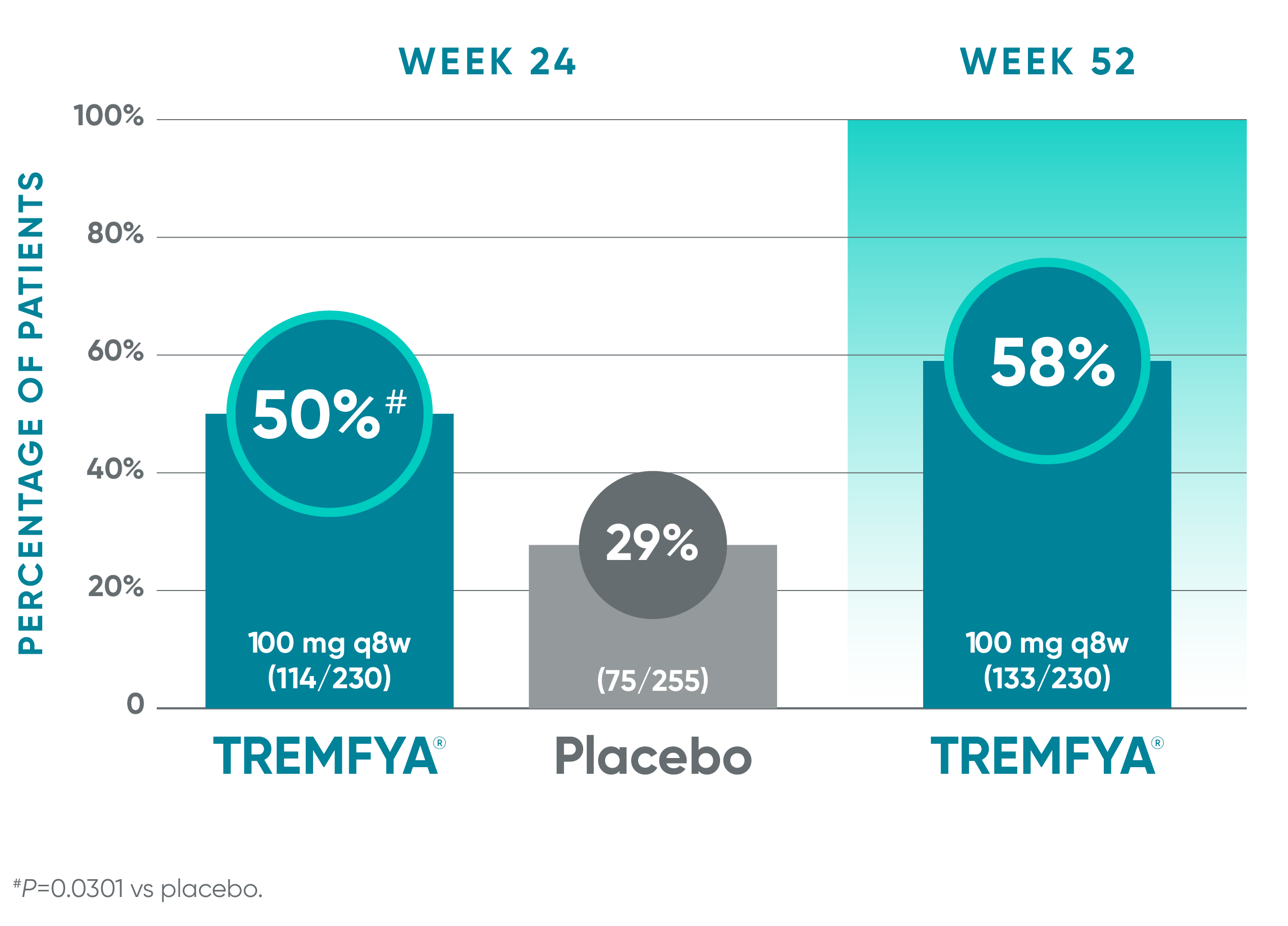
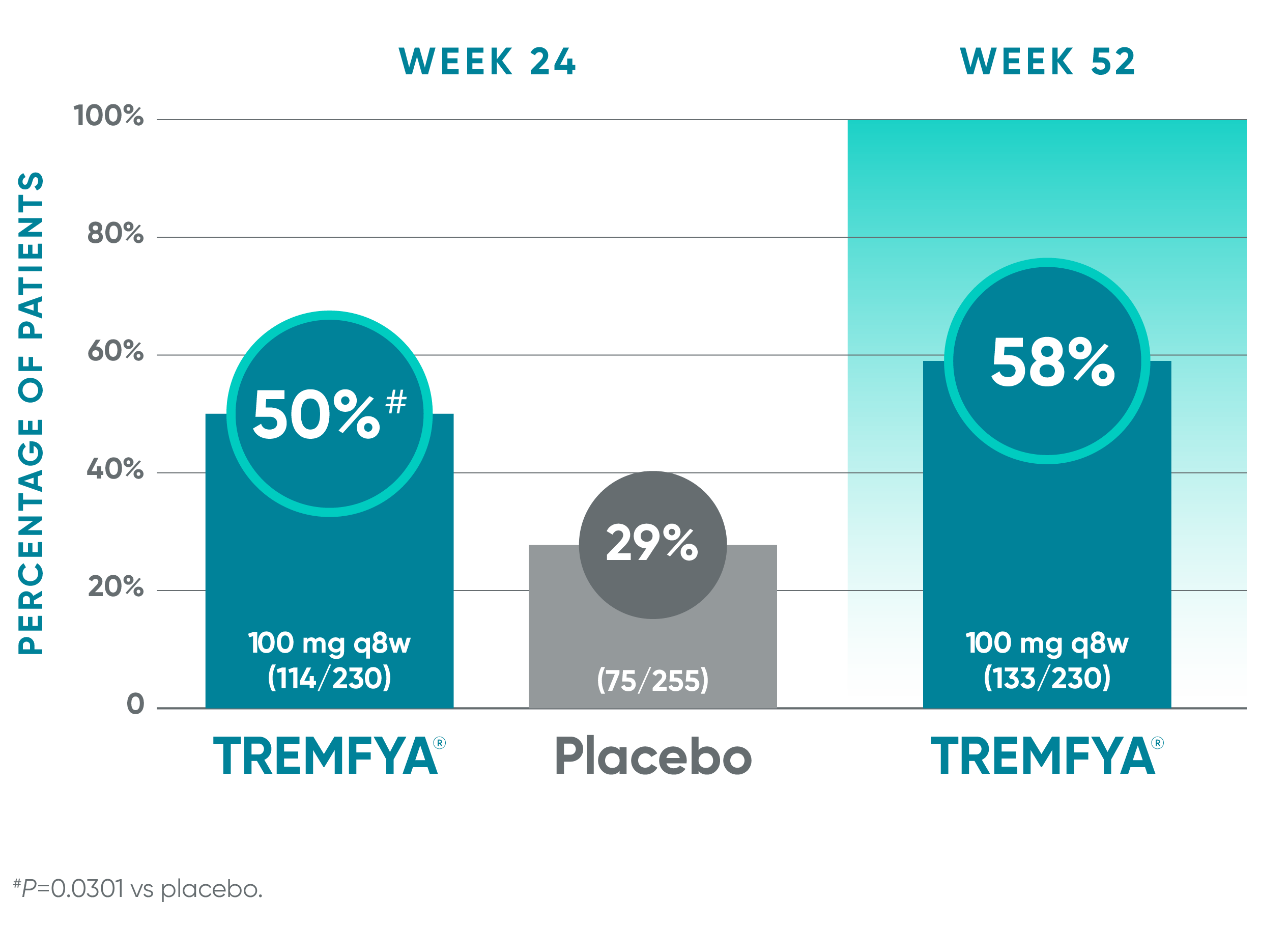
1 in 2 patients experienced complete resolution of enthesitis at Week 241-4*
Pooled data from DISCOVER 1 and DISCOVER 2: Resolution of enthesitis (LEI score=0) at Weeks 24 (NRI) and 52 (NRI post hoc analysis)†‡§
Blinded, placebo-controlled phase (NRI)
Open-label active treatment, NRI post hoc analysisII¶


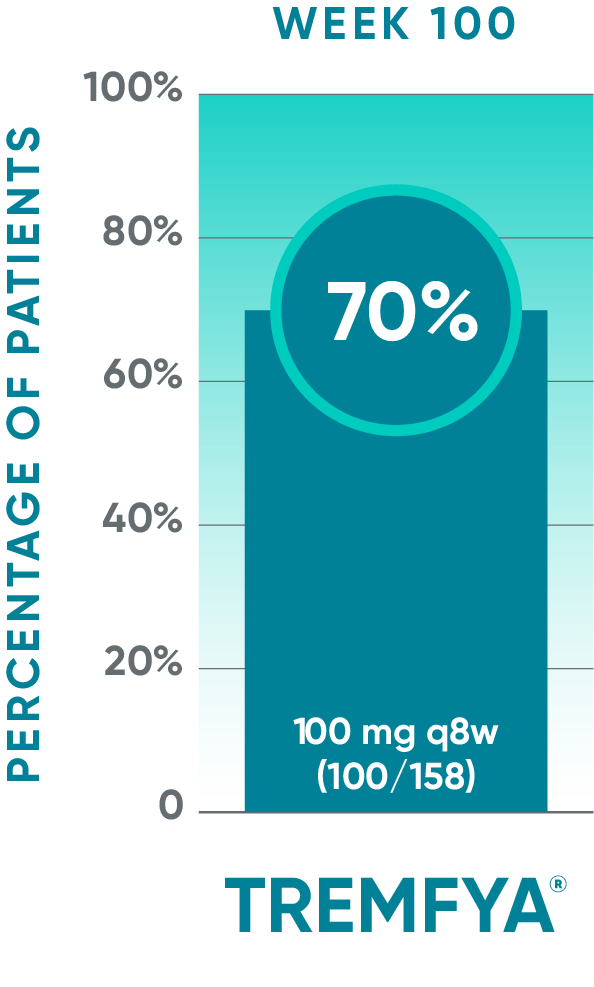
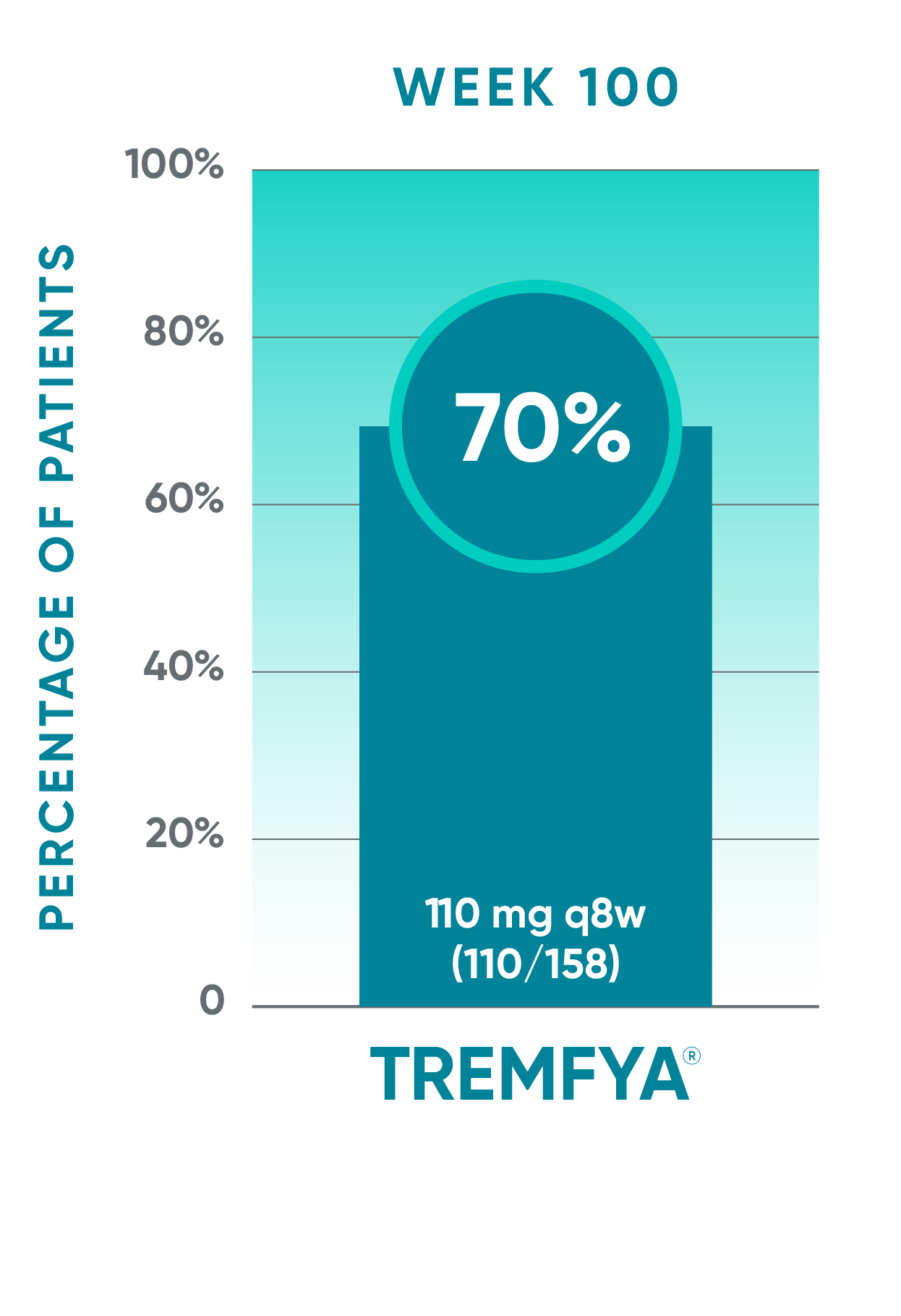
DISCOVER 2 only: Resolution of enthesitis (LEI score=0) at Week 100 (NRI post hoc analysis)†§
Open-label active treatment, NRI post hoc analysisII¶


*The same patients may not have responded at each time point.
†Among patients with LEI enthesitis score >0 at baseline.
‡Through Week 24, patients were considered to be nonresponders after meeting treatment failure criteria: discontinued study agent for any reason, terminated study participation for any reason, initiated or increased the dose of disease-modifying antirheumatic drugs (DMARDs) or oral corticosteroids over baseline for PsA, or initiated protocol-prohibited medications/therapies for PsA. After Week 24, treatment failure rules were not applied.
§Patients with missing data were considered nonresponders.
||After Week 24, patients and doctors knew that all patients were on TREMFYA® (open label with a blinded dosing interval), which may have affected the results.
¶The prespecified as-observed analyses at Week 52 and Week 100 are not shown.
LEI=Leeds Enthesitis Index.
References: 1. Data on file. Janssen Biotech, Inc. 2. TREMFYA® (guselkumab) [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc. 3. McInnes IB, Rahman P, Gottlieb AB, et al. Efficacy and safety of guselkumab, an interleukin-23p19-specific monoclonal antibody, through one year in biologic-naïve patients with psoriatic arthritis. Arthritis Rheumatol. 2021;73(4):604-616. 4. McInnes IB, Rahman P, Gottlieb AB, et al. Efficacy and safety of guselkumab, a monoclonal antibody specific to the p-19 subunit of interleukin-23, through 2 years: results from a phase 3, randomized, double-blind, placebo-controlled study conducted in biologic-naïve patients with active psoriatic arthritis. Presented at: Innovations in Dermatology 2021; Virtual; March 16-20, 2021.
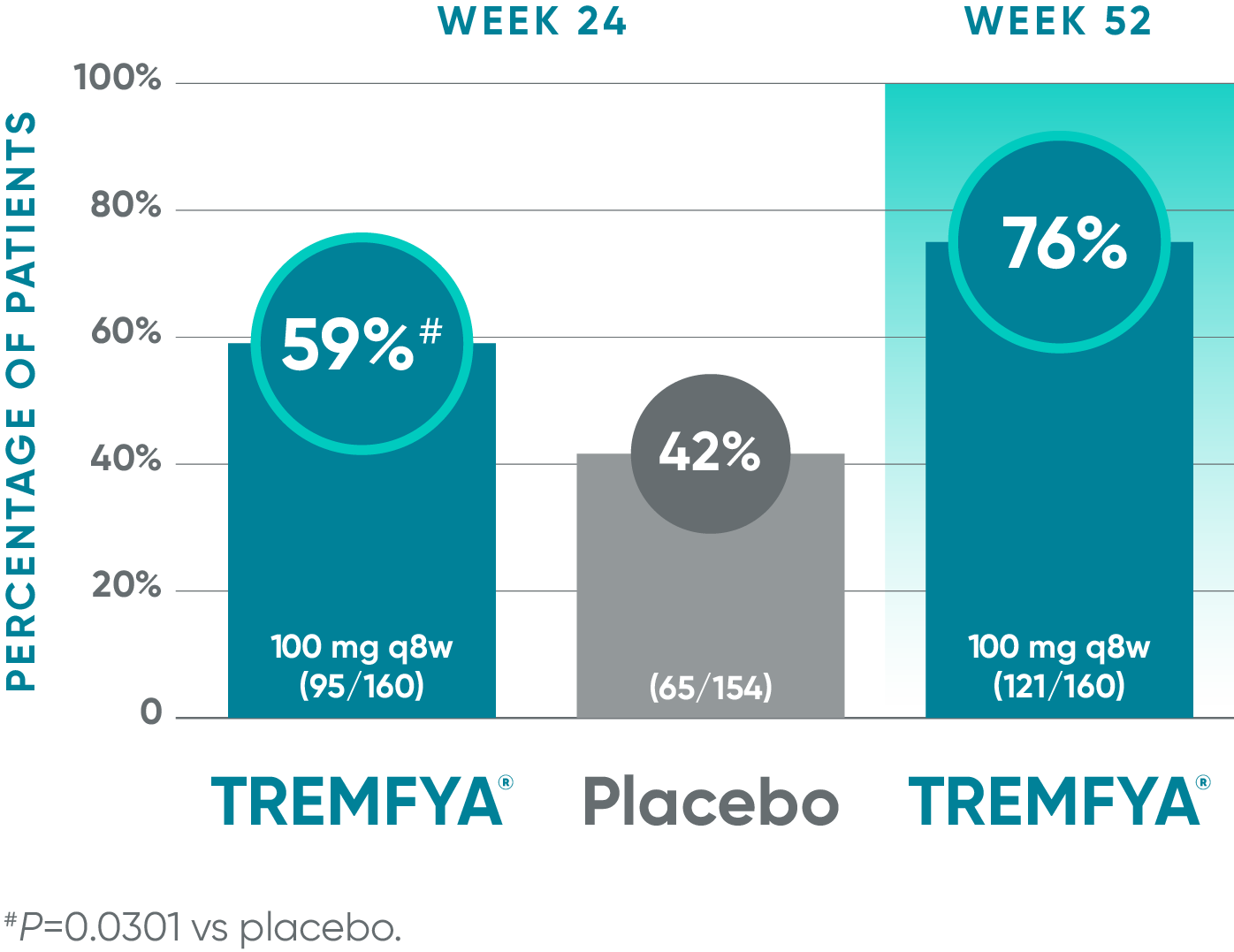
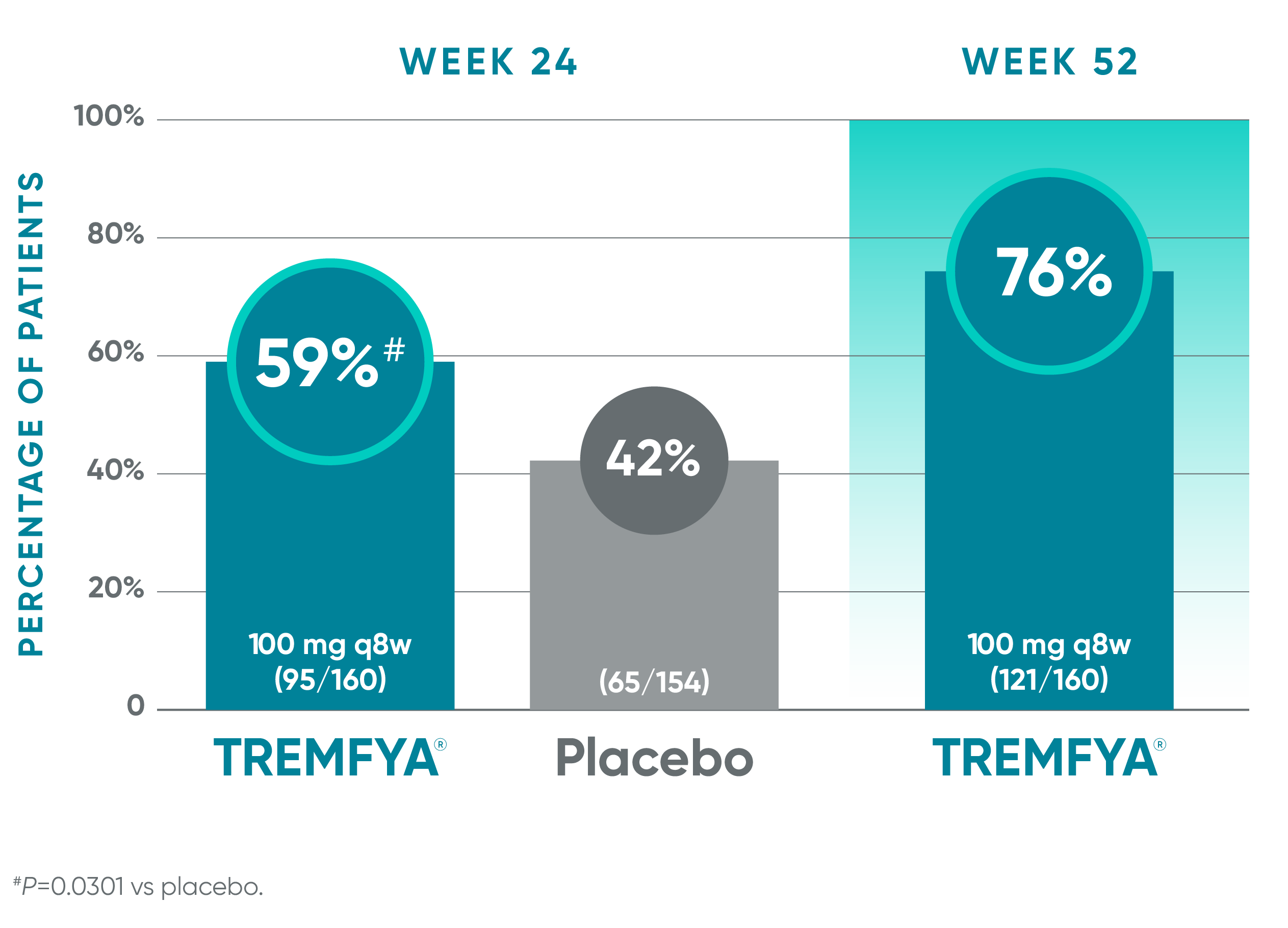
Nearly 6 out of 10 patients experienced complete resolution of dactylitis at Week 241-3*†
Pooled data from DISCOVER 1 and DISCOVER 2: Resolution of dactylitis (dactylitis score=0) at Weeks 24 (NRI) and 52 (NRI post hoc analysis)†‡§
Blinded, placebo-controlled phase (NRI)
Open-label active treatment, NRI post hoc analysisII¶


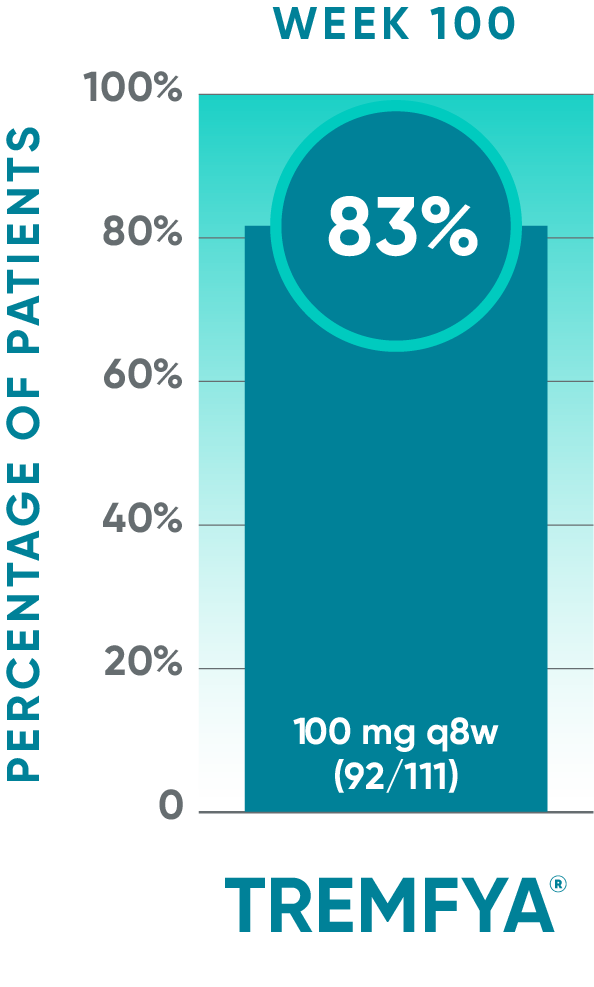
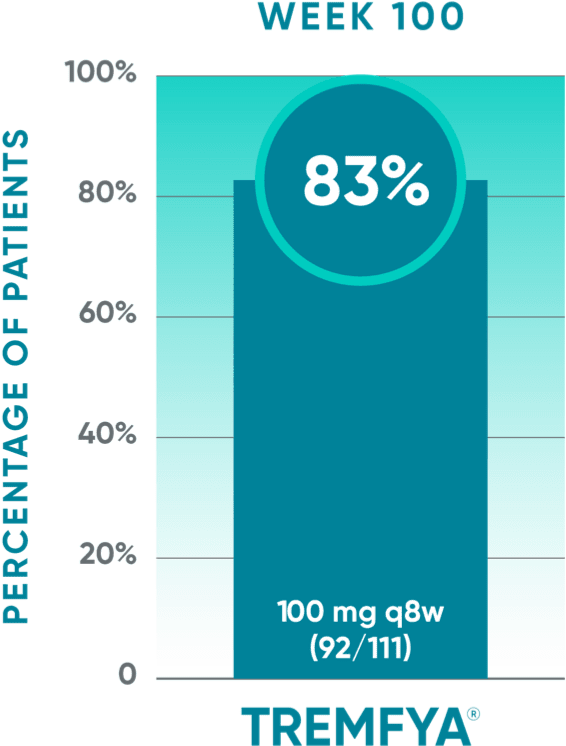
DISCOVER 2 only: Resolution of dactylitis (dactylitis score=0) at Week 100 (NRI post hoc analysis)*‡§
Open-label active treatment, NRI post hoc analysisII¶


*The same patients may not have responded at each time point.
†Among patients with dactylitis at baseline.
‡Through Week 24, patients were considered to be nonresponders after meeting treatment failure criteria: discontinued study agent for any reason, terminated study participation for any reason, initiated or increased the dose of disease-modifying antirheumatic drugs (DMARDs) or oral corticosteroids over baseline for PsA, or initiated protocol-prohibited medications/therapies for PsA. After Week 24, treatment failure rules were not applied.
§Patients with missing data were considered nonresponders.
||After Week 24, patients and doctors knew that all patients were on TREMFYA® (open label with a blinded dosing interval), which may have affected the results.
¶The prespecified as-observed analyses at Week 52 and Week 100 are not shown.
References: 1. TREMFYA® (guselkumab) [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc. 2. McInnes IB, Rahman P, Gottlieb AB, et al. Efficacy and safety of guselkumab, a monoclonal antibody specific to the p-19 subunit of interleukin-23, through 2 years: results from a phase 3, randomised, double-blind, placebo-controlled study conducted in biologic-naive patients with active psoriatic arthritis. Presented at: Innovations in Dermatology 2021; Virtual; March 16-20, 2021. 3. Data on file. Janssen Biotech, Inc.
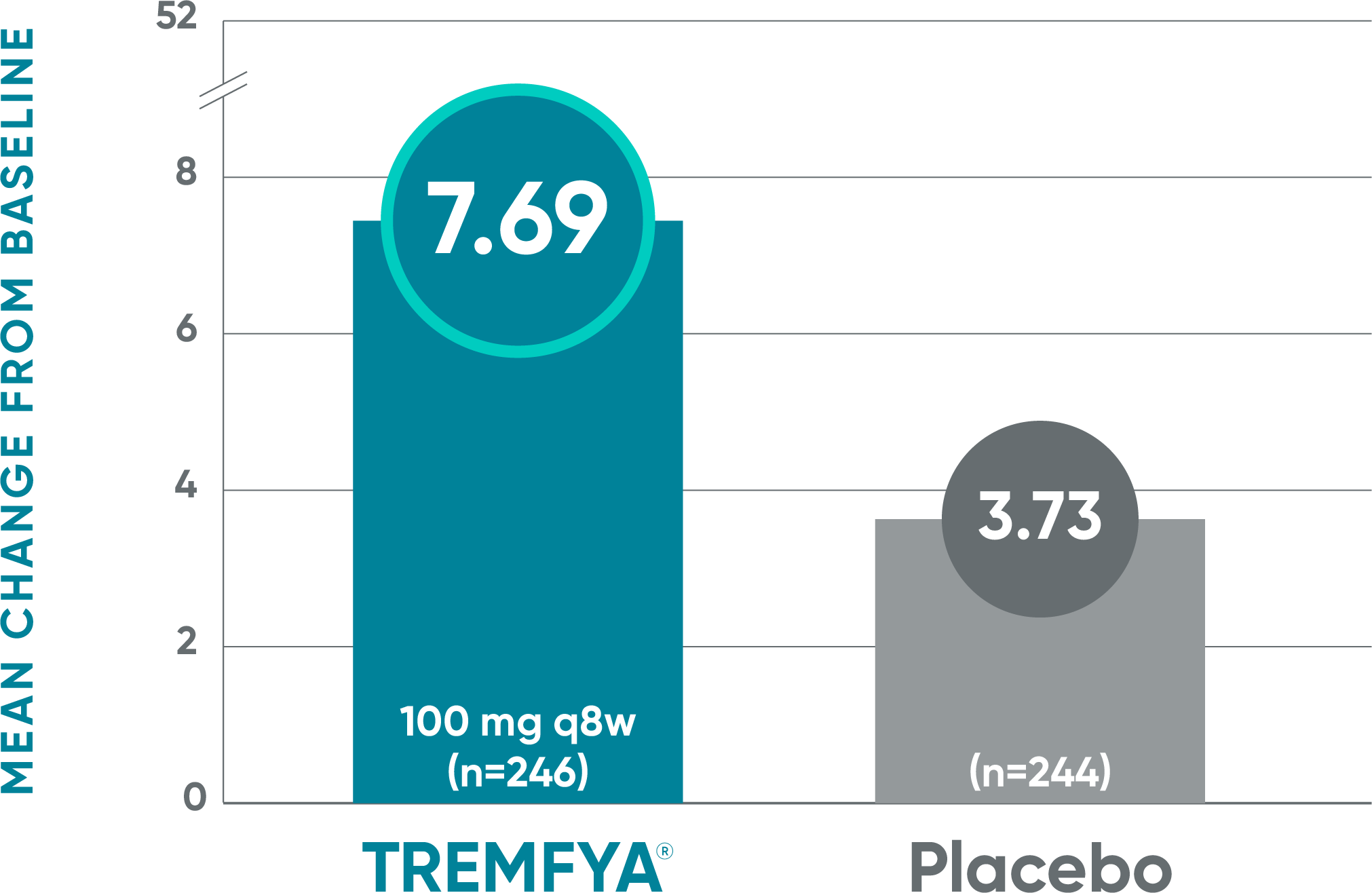
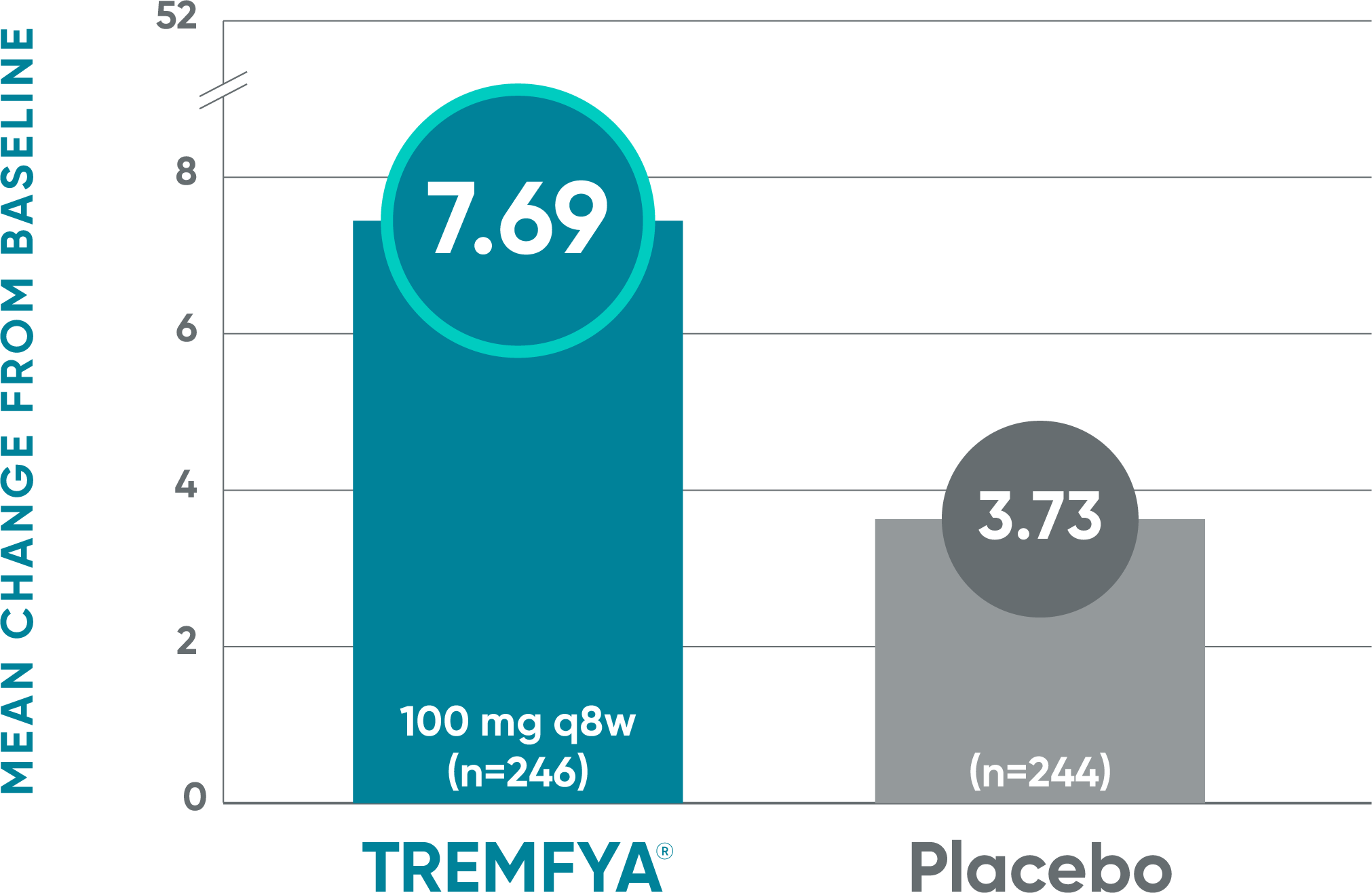
Treatment with TREMFYA® resulted in improvement in fatigue as measured by FACIT-F1,2
DISCOVER 2: Mean change from baseline in FACIT-F score at Week 24 (NRI)1*†‡
Blinded, placebo-controlled phase


In DISCOVER 1 at Week 24
- The mean change from baseline in FACIT-F score was 5.76 for patients receiving TREMFYA® q8w (n=127) vs 2.15 for patients receiving placebo (n=126)1*†‡
The FACIT-F endpoints in DISCOVER 1 and DISCOVER 2 were not adjusted for multiplicity. Therefore, statistical significance has not been established.1
*Through Week 24, patients were considered to have no improvement (change=0) after meeting treatment failure criteria: discontinued study agent for any reason, terminated study participation for any reason, initiated or increased the dose of disease-modifying antirheumatic drugs (DMARDs) or oral corticosteroids over baseline for PsA, or initiated protocol-prohibited medications/therapies for PsA. After Week 24, treatment failure rules were not applied.
†Change from baseline, if missing, was considered to have no improvement (change=0) after patients discontinued study treatment due to any reason.
‡Patients with missing data were considered nonresponders.
FACIT-F=Functional Assessment of Chronic Illness Therapy-Fatigue; NRI=nonresponder imputation.
FACIT-Fatigue (FACIT-F) measures a patient's level of fatigue and tiredness over the last 7 days through a questionnaire consisting of 13 questions. Lower scores reflect more severe fatigue.1
References: 1. Data on file. Janssen Biotech, Inc. 2. TREMFYA® (guselkumab) [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc.


