For US Healthcare Professionals
I am a
Rheumatology Specialist
For US Healthcare Professionals
Full Prescribing InformationSign-up Portal
Explore coverage options in your area
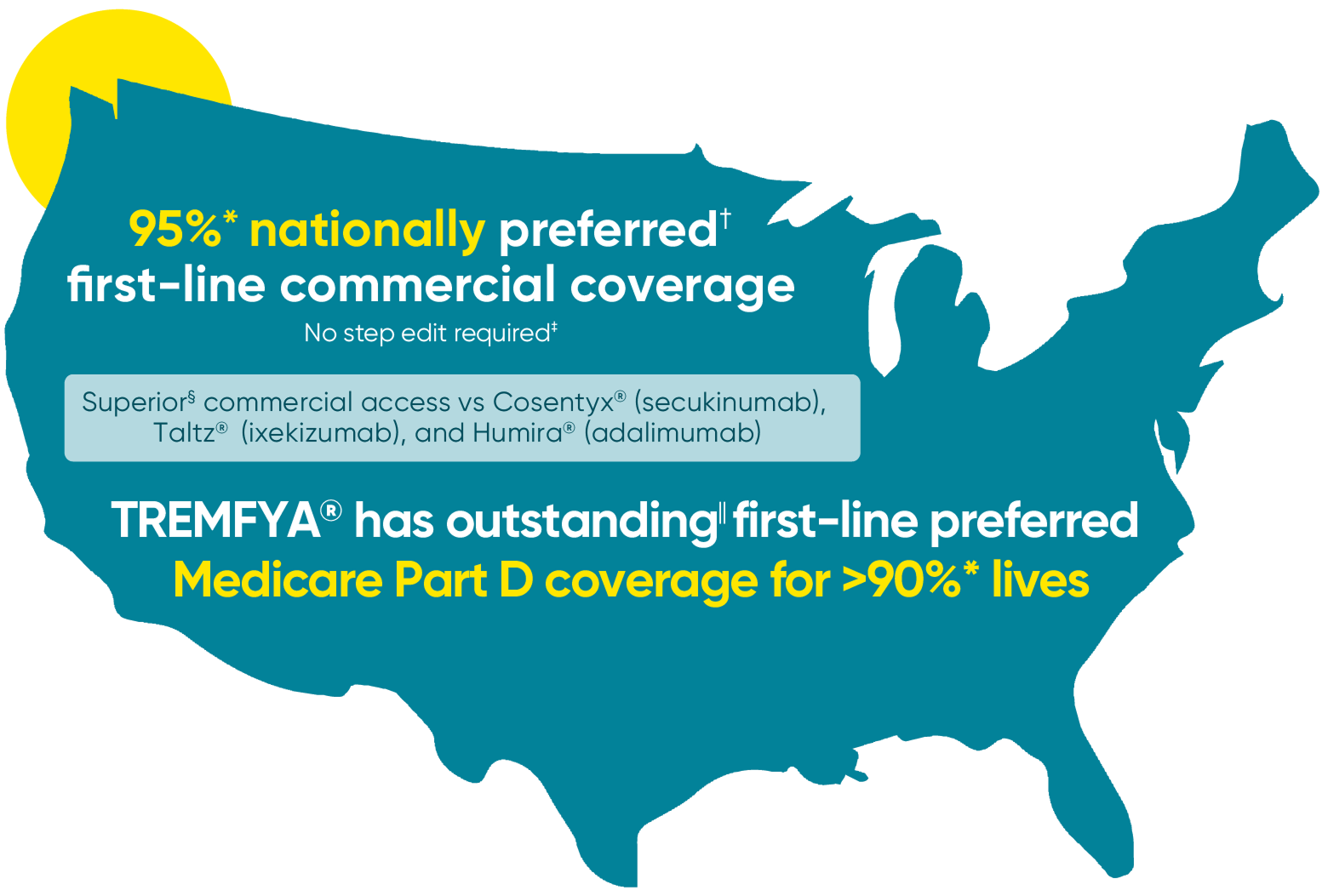
View Covered Medicare Part D Plans
DownloadThis information does not provide advice or guarantee coverage or payment. Legal requirements and plan information can be updated frequently. We strongly recommend contacting the plan for more information about current coverage, restrictions, or prerequisites that may apply.
Source: Managed Markets Insight & Technology, LLCTM, a trademark of MMIT, as of September 2025. Collected as of 09/25 and may change.
Data on file, as of 09/2025. Collected as of 09/25 and may change.
*These percentages may not represent 100% of formulary lives due to data limitations.
†“Preferred” means TREMFYA® can be accessed first-line (ie, step therapy is not required) and its formulary status is better than or equivalent to other products in the class.
‡“No Step Therapy”: This plan does not require the patient to step through and fail an alternate product before this product is dispensed.
§“Superior” means difference is greater than 10%. The information provided does not imply comparable safety or efficacy between products and only represents access information. Indicated trademarks are the registered trademarks of their respective owners. Please refer to each product’s Prescribing Information for indication(s), recommended dosing, and administration.
II“Outstanding” means >90% number of covered lives.

