TREMFYA® is the ONLY* IL-23i proven to significantly inhibit structural damage progression1,2†
IN ADULT PATIENTS WITH ACTIVE PsA
APEX was conducted to build upon the DISCOVER program, which showed a reduction in structural damage progression but was not statistically significant3,4
DISCOVER 2: Change from baseline in mvdH-S score: Blinded, placebo-controlled (Week 24)
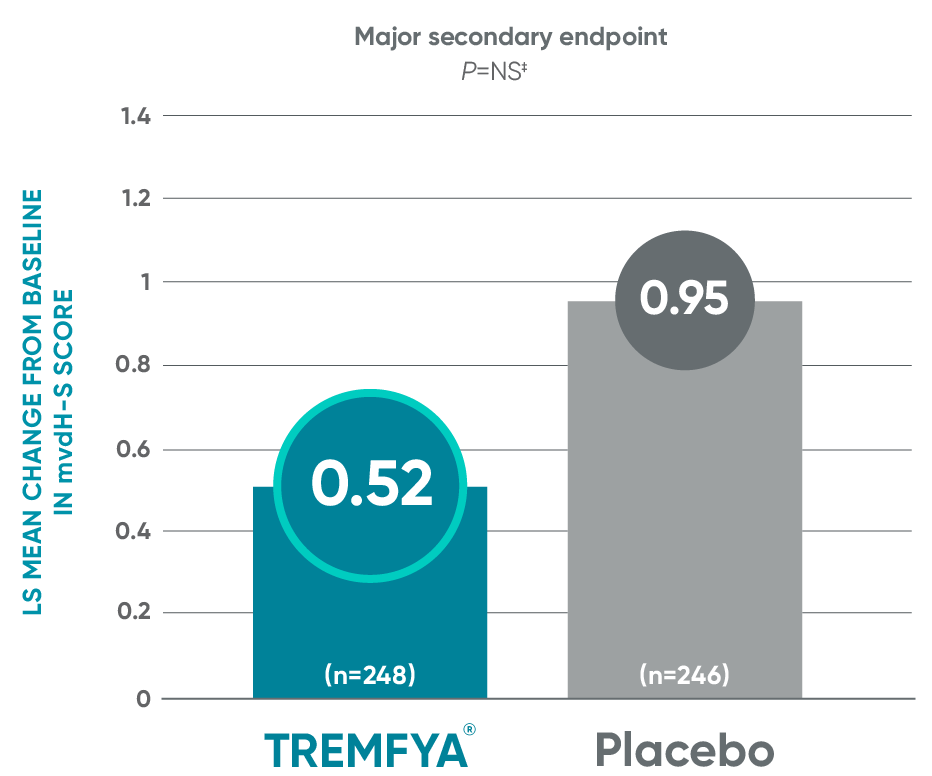
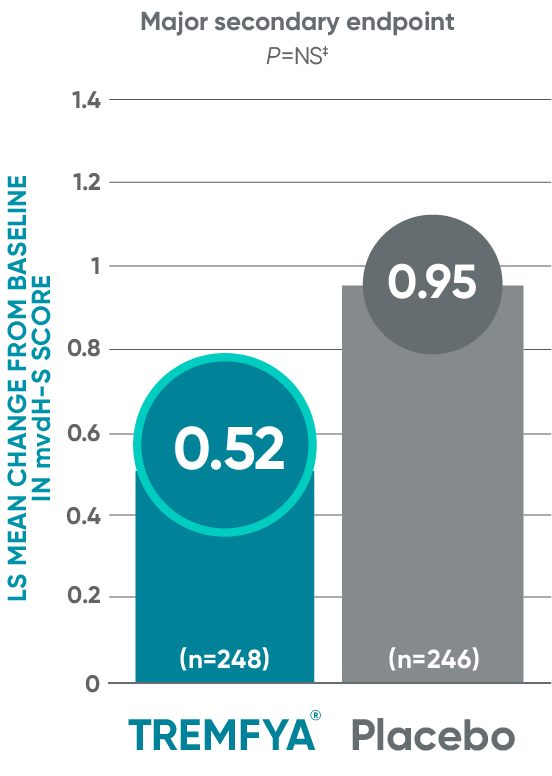
The results are not statistically significant; therefore, treatment effect for inhibition of structural damage has not been established.
Patients received TREMFYA® 100 mg SC at Week 0, Week 4, and every 8 weeks thereafter.
Treatment failure rules were not applied, and missing data were assumed to be missing at
random and were imputed using
multiple imputation.
IL-23i=interleukin-23 inhibitor; LS=least squares;
mvdH-S=modified van der Heijde-Sharp; NS=not significant; PsA=psoriatic arthritis; SC=subcutaneous.
*Based on approved IL-23 inhibitors for active PsA as of August 2025.
Source: IQVIA Weekly LAAD as of 08/21/25 X Free goods; Total PsA EqU share for Rheumatologists.
†Major secondary endpoint in a phase 3b study.
‡P value is not significant.
IN ADULT PATIENTS WITH ACTIVE PsA
Joint improvement with TREMFYA®, consistent with pivotal trials1,2
APEX: ACR20/50/70 responses: Blinded, placebo-controlled (Week 24)*
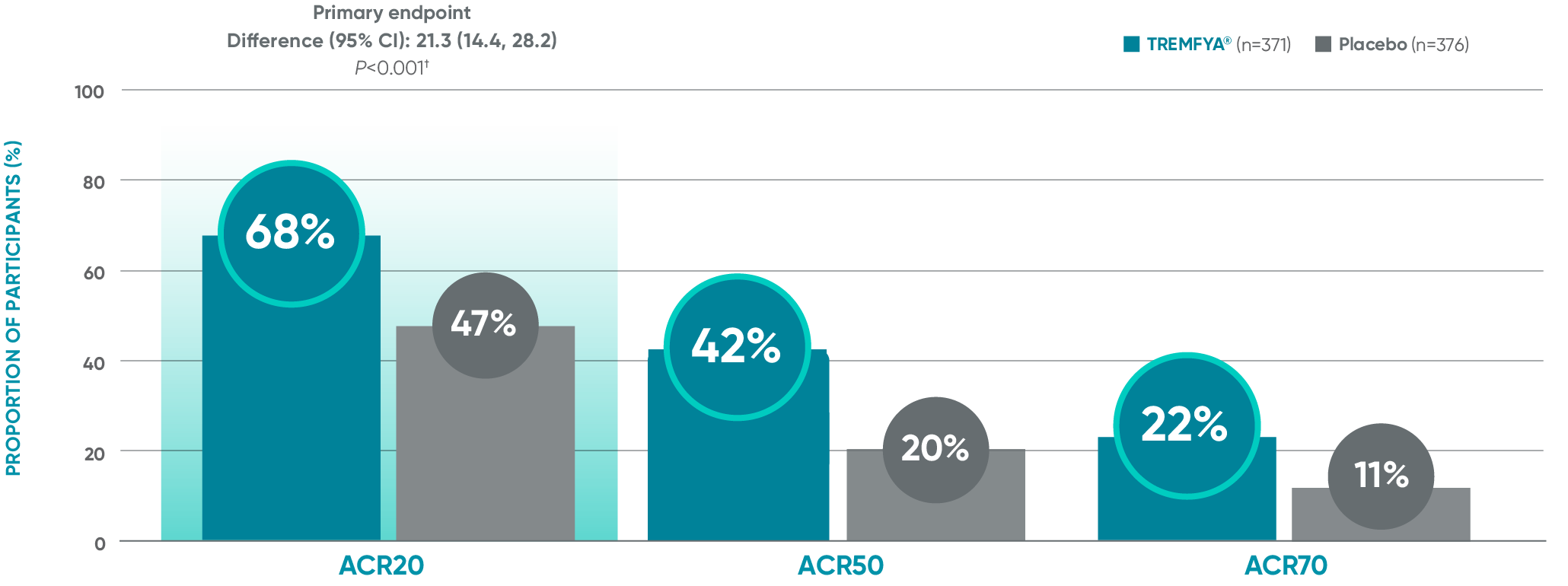
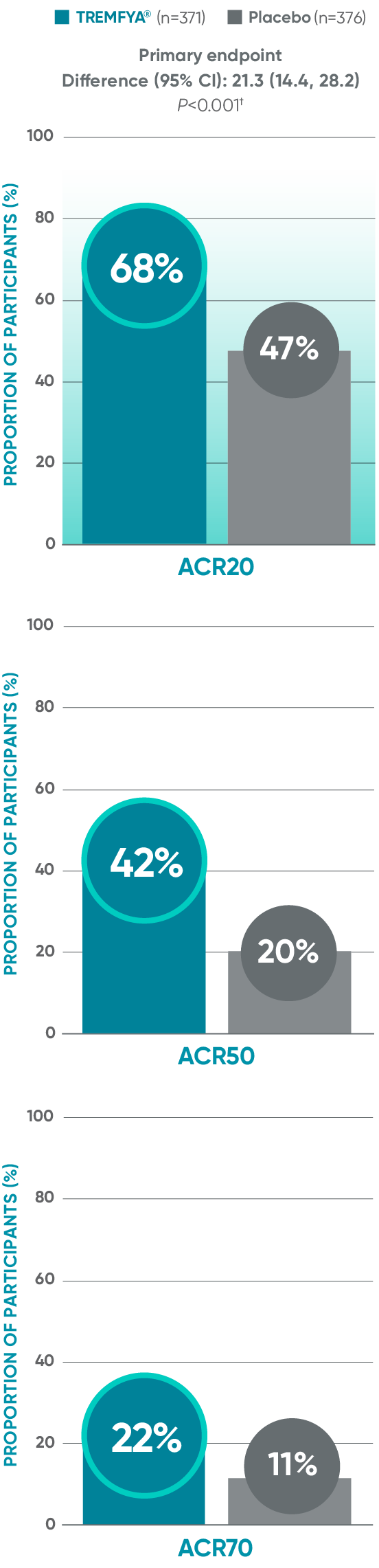
ACR50 and ACR70 responses at Week 24 were not adjusted for multiplicity; therefore, statistical significance has not been established.
Primary endpoint in pivotal studies: At Week 24, adult patients with active PsA receiving TREMFYA® demonstrated a greater clinical response
in ACR20 compared to placebo, in both the DISCOVER 1 (52% vs 22%) and DISCOVER 2 (64% vs 33%) trials, respectively (P<0.0001).3,5,6
ACR20=20% improvement in American College of Rheumatology composite measures of arthritis; ACR50=50% improvement in American College
of Rheumatology composite measures of arthritis; ACR70=70% improvement in American College of Rheumatology composite measures of arthritis;
CMH=Cochran-Mantel-Haenszel; mFAS=modified full analysis set.
*Efficacy analyses are from the mFAS, which included all randomized patients, excluding those from Ukraine sites rendered unable to
support key study operations due to major disruptions.
†The primary endpoint P value is multiplicity controlled using a fixed sequence testing procedure and can be used to determine statistical
significance. Statistics are based on the CMH test across multiply imputed datasets.
GIVE YOUR ADULT PATIENTS WITH ACTIVE PsA THE CHANCE FOR
Joint preservation with TREMFYA®1,2
Significant (2.5x) reduction in structural damage progression vs placebo
APEX: Change in total mvdH-S score: Blinded, placebo-controlled (Week 24)*†
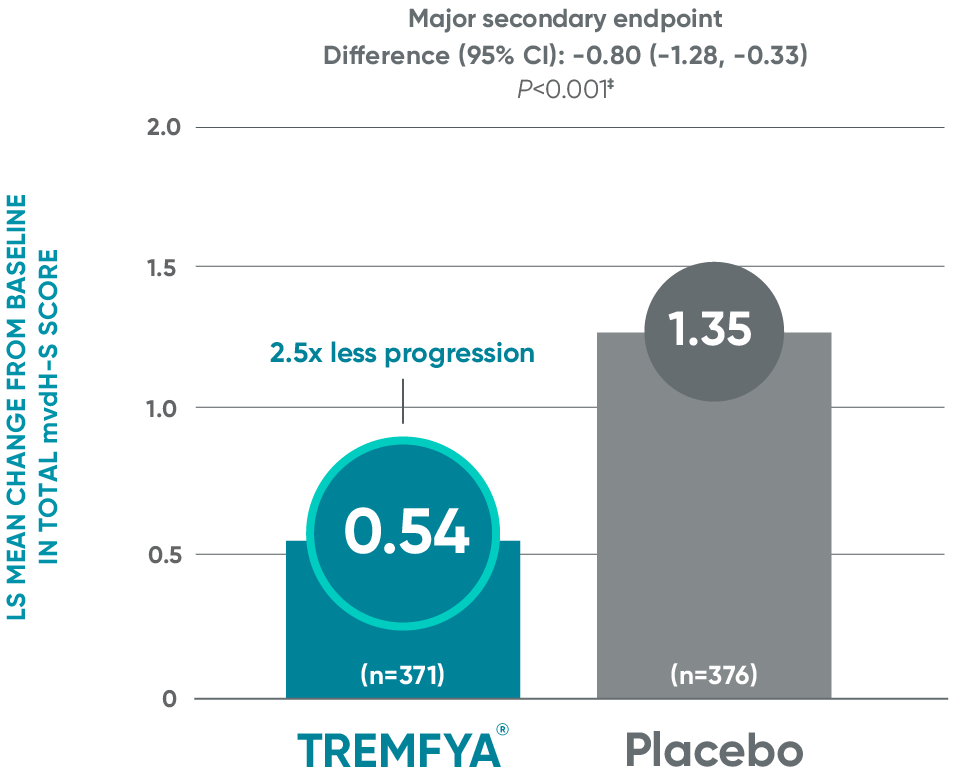
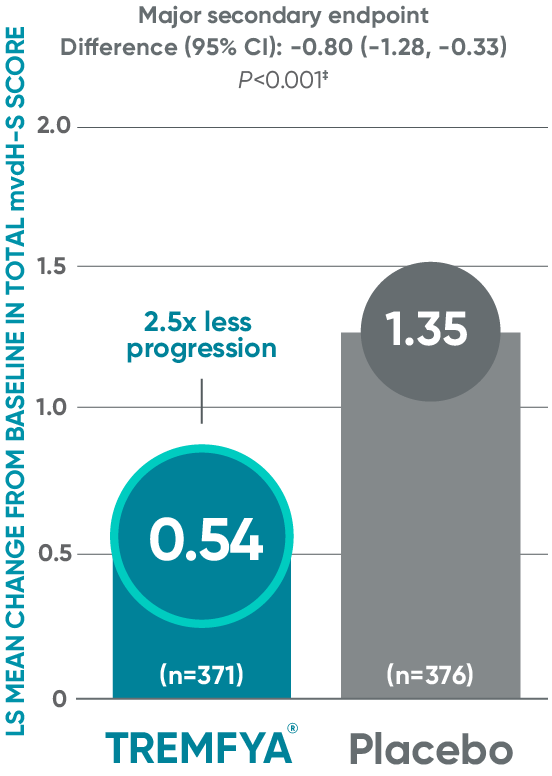
ANCOVA=analysis of covariance; ND/MD=natural disaster/
major disruption.
*Efficacy analyses are from the mFAS, which included all randomized patients, excluding those from Ukraine sites rendered unable to
support key study operations due to major disruptions.
†Statistics are based on ANCOVA. Missing data and data impacted by ND/MD were imputed using multiple imputation.
‡Major secondary endpoint P value is multiplicity controlled using a fixed sequence testing procedure and can be used to determine
statistical significance. Statistics are based on ANCOVA across multiply imputed datasets.
APEX: Erosion and joint space narrowing scores with TREMFYA at Week 241,2*†‡
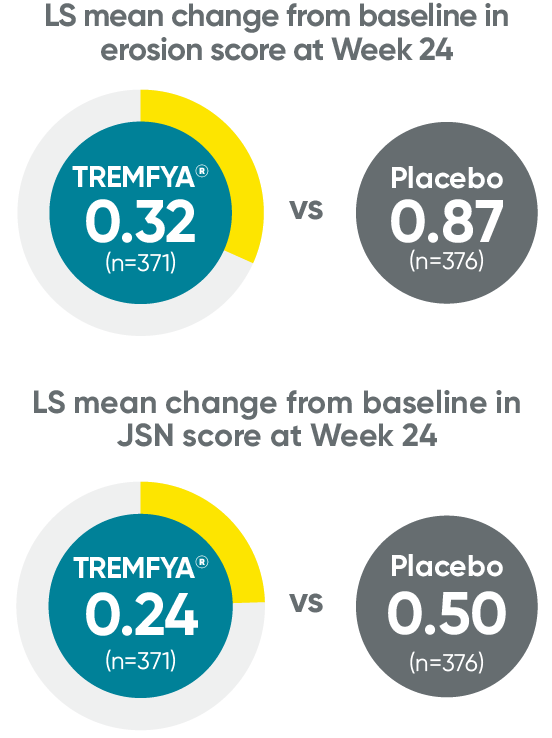
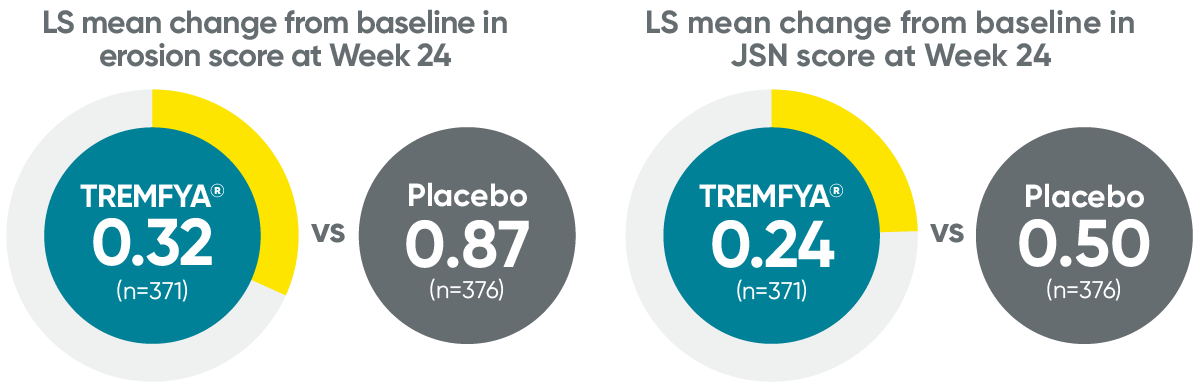
†P values for erosion score and joint space narrowing score are not multiplicity controlled; they are considered nominal and may not be used to claim statistical significance.
‡Based on ANCOVA on multiply imputed data.
JSN=joint space narrowing.
*Efficacy analyses are from the mFAS, which included all randomized patients, excluding those from Ukraine sites rendered unable to
support key study operations due to major disruptions.
‡Based on ANCOVA on multiply imputed data.
JSN=joint space narrowing.
*Efficacy analyses are from the mFAS, which included all randomized patients, excluding those from Ukraine sites rendered unable to
support key study operations due to major disruptions.
APEX: Proven safety profile through
Week 241,2
Adverse events reported in the placebo-controlled phase through Week 24 in APEX
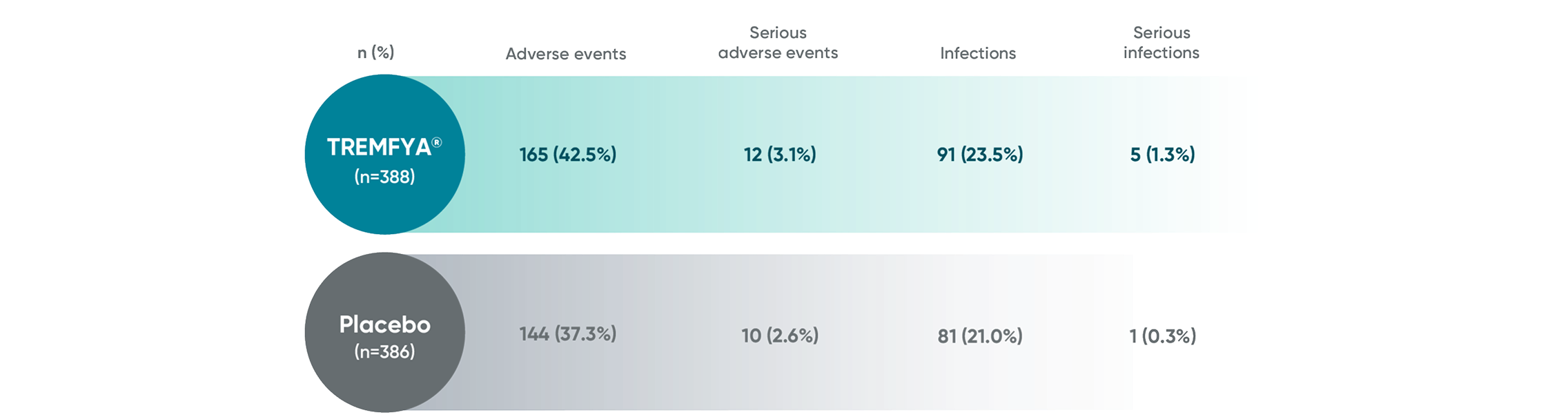
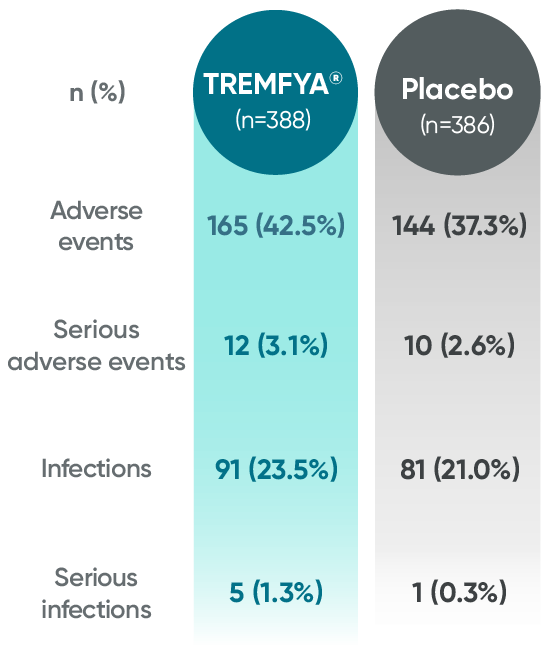
No new safety signals were seen through Week 24 in APEX1
References: 1. Mease PJ, Ritchlin CT, Coates LC, et al. Inhibition of structural damage progression with guselkumab, a selective IL-23i, in participants with active PsA: results through week 24 of the phase 3b, randomized, double-blind, placebo-controlled APEX study. Oral presentation at: European Alliance of Associations for Rheumatology (EULAR) 2025 Congress; June 11-14, 2025; Barcelona, Spain. 2. Mease PJ, Ritchlin CT, Coates LC, et al. Inhibition of structural damage progression with guselkumab, a selective IL-23i, in participants with active PsA: results through week 24 of the phase 3b, randomized, double-blind, placebo-controlled APEX study. Abstract presented at: European Alliance of Associations for Rheumatology (EULAR) 2025 Congress; June 14, 2025; Barcelona, Spain. Late-Breaking Abstracts Session II. 3. Mease PJ, Rahman P, Gottlieb AB, et al. Guselkumab in biologic-naïve patients with active psoriatic arthritis (DISCOVER-2): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395(10230):1126-1136.
4. Ritchlin CT, Coates LC, Mease PJ, et al. The effect of guselkumab on inhibiting radiographic progression in patients with active psoriatic arthritis: study protocol for APEX, a Phase 3b, multicenter, randomized, double-blind, placebo-controlled trial. Trials. 2023;24(1):22. 5. TREMFYA® (guselkumab) [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc. 6. Deodhar A, Helliwell PS, Boehncke W-H, et al. Guselkumab in patients with active psoriatic arthritis who were biologic-naïve or had previously received TNFα inhibitor treatment (DISCOVER 1): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395(10230):1115-1125.


