IN ADULT PATIENTS WITH ACTIVE PSORIATIC ARTHRITIS (PsA) OR MODERATE TO SEVERE PLAQUE PSORIASIS (PsO)
TREMFYA® has optimized 8-week dosing and the One-Press patient-controlled injector
TREMFYA® is administered as a 100-mg subcutaneous injection once every 8 weeks, after 2 starter doses at Weeks 0 and 41
injections per year*


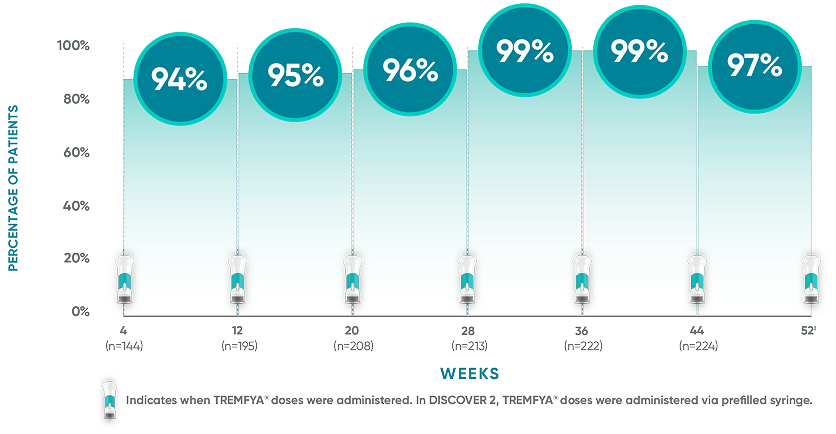
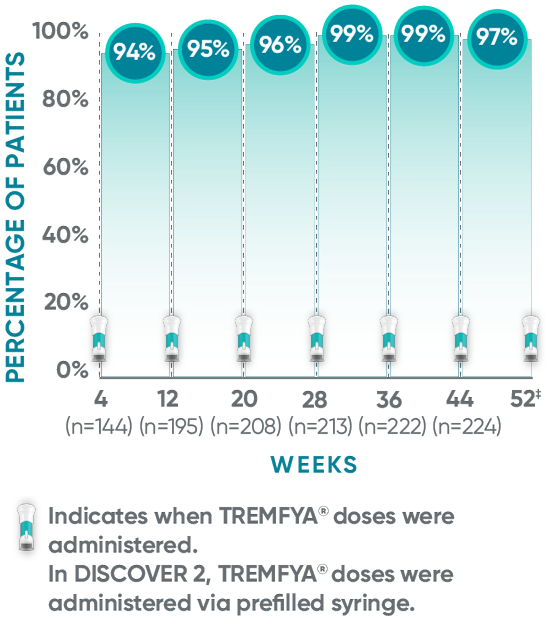
The TREMFYA® 8-week dosing schedule was researched in clinical trials1,2
PsA/PsO dosage forms
- Prefilled syringe 100 mg/mL (NDC: 57894-640-01)
- One-Press patient-controlled injector 100 mg/mL (NDC: 57894-640-11)
Pretreatment Evaluation: Evaluate for tuberculosis (TB) infection, evaluate liver enzymes and bilirubin levels (in UC and CD); if clinically indicated, evaluate liver enzymes and bilirubin levels (in PsO and PsA). Complete all age-appropriate vaccinations according to current immunization guidelines.
Monitor: For signs and symptoms of active TB during and after treatment with TREMFYA®. For the treatment of CD or UC, evaluate liver enzymes and bilirubin levels for at least 16 weeks of treatment, and periodically thereafter according to routine patient management. In patients with PsO or PsA, if clinically indicated, evaluate liver enzymes and bilirubin levels periodically according to routine patient management.
TREMFYA® is intended for use under the guidance and supervision of a healthcare professional. After proper training in subcutaneous injection technique, adults may self-inject. Pediatric self-administration is not recommended. Administration of TREMFYA® to pediatric patients should be performed by a healthcare provider or by a caregiver who has received training and demonstrated proper subcutaneous injection technique.
In active PsA, TREMFYA® can be used alone or in combination with a conventional DMARD (eg, methotrexate).
CD=Crohn's disease; DMARD=disease-modifying antirheumatic drug; NDC=National Drug Code; UC=ulcerative colitis.
*Frequency of maintenance injections for TREMFYA® after the first year.