Post hoc analysis: pooled data from DISCOVER 1 and DISCOVER 2: LS mean change from baseline in BASDAI at Week 24 and Week 52*†‡
Data from DISCOVER 2 only: LS mean change from baseline in BASDAI at Week 100
For US Healthcare Professionals
I am a
Rheumatology Specialist
For US Healthcare Professionals
Full Prescribing InformationDurable joint improvement at 2 years with TREMFYA®1-5*†‡§ǁ
DISCOVER 2 ACR20/50/70: Open-label active treatment, NRI post hoc analysis (Weeks 24-100)
ACR50 and ACR70 at Week 24 were not part of the sequential testing procedure but were prespecified to be tested upon achieving statistical significance for ACR20 at Week 24.
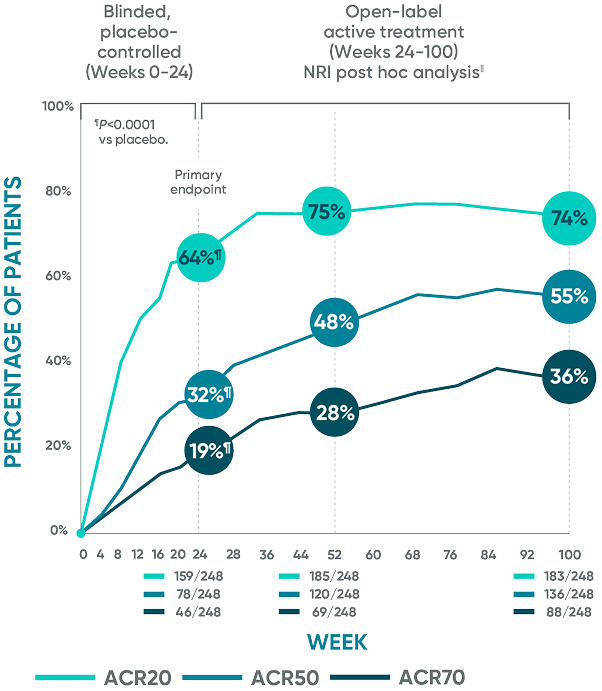
The same patients may not have responded at each time point.
DISCOVER 2: Week 24: Blinded, placebo-controlled NRI¶
ACR20 placebo response:
33% (81/246)
ACR50 placebo response:
14% (35/246)
ACR70 placebo response:
4% (10/246)
ACR20=20% improvement in American College of Rheumatology composite measures of arthritis;
ACR50=50% improvement in American College of Rheumatology composite measures of arthritis;
ACR70=70% improvement in American College of Rheumatology composite measures of arthritis; NRI=nonresponder imputation.
*Year 2 represents Week 100.
†Through Week 24, patients were considered to be nonresponders after meeting treatment failure criteria (see study designs). After Week 24, treatment failure rules were not applied.
‡Patients with missing data were considered nonresponders.
§After Week 24, patients and doctors knew that all patients were on TREMFYA® (open label with a blinded dosing interval), which may have affected the results.
||The DISCOVER 2 prespecified as-observed analysis from Weeks 24 to 100 is not shown.
References: 1. TREMFYA® (guselkumab) [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc. 2. Data on file. Janssen Biotech, Inc. 3. Mease PJ, Rahman P, Gottlieb AB, et al. Guselkumab in biologic-naïve patients with active psoriatic arthritis (DISCOVER 2): a double-blind, randomized, placebo-controlled phase 3 trial. Lancet. 2020;395(10230):1126-1136. 4. McInnes IB, Rahman P, Gottlieb AB, et al. Long-term efficacy and safety of guselkumab, a monoclonal antibody specific to the p19 subunit of interleukin-23, through two years: results from a phase III, randomized, double-blind, placebo-controlled study conducted in biologic-naïve patients with active psoriatic arthritis. Arthritis Rheumatol. 2022;74(3):475-485. 5. McInnes IB, Rahman P, Gottlieb AB, et al. Efficacy and safety of guselkumab, an interleukin-23p19-specific monoclonal antibody, through one year in biologic-naïve patients with psoriatic arthritis. Arthritis Rheumatol. 2021;73(4):604-616.
Complete resolution of enthesitis and dactylitis at 2 years with TREMFYA®1-5*†‡§ǁ¶#
Pooled data from DISCOVER 1 & DISCOVER 2 at Week 24 and 52 and from only DISCOVER 2 at Week 100

Complete resolution of enthesitis and dactylitis are defined as LEI score=0 and dactylitis score=0, respectively.
LEI=Leeds Enthesitis Index; NRI=nonresponder imputation.
*The same patients may not have responded at each time point.
†Year 2 represents Week 100.
‡After Week 24, patients and doctors knew that all patients were on TREMFYA® (open label with a blinded dosing interval), which may have affected the results.
§Among patients with dactylitis score >0 at baseline.
||Among patients with LEI enthesitis score >0 at baseline.
¶Through Week 24, patients were considered to be nonresponders after meeting treatment failure criteria (see study designs). After Week 24, treatment failure rules were not applied.
#Patients with missing data were considered nonresponders.
††The DISCOVER 2 prespecified as-observed analysis from Weeks 24 to 100 is not shown.
References: 1. TREMFYA® (guselkumab) [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc. 2. Data on file. Janssen Biotech, Inc. 3. Mease PJ, Rahman P, Gottlieb AB, et al. Guselkumab in biologic-naïve patients with active psoriatic arthritis (DISCOVER 2): a double-blind, randomized, placebo-controlled phase 3 trial. Lancet. 2020;395(10230): 1126-1136. 4. McInnes IB, Rahman P, Gottlieb AB, et al. Long-term efficacy and safety of guselkumab, a monoclonal antibody specific to the p19 subunit of interleukin-23, through two years: results from a phase III, randomized, double-blind, placebo-controlled study conducted in biologic-naïve patients with active psoriatic arthritis. Arthritis Rheumatol. 2022;74(3):475-485. 5. McInnes IB, Rahman P, Gottlieb AB, et al. Efficacy and safety of guselkumab, an interleukin-23p19-specific monoclonal antibody, through one year in biologic-naïve patients with psoriatic arthritis. Arthritis Rheumatol. 2021;73(4):604-616.
TREMFYA®
demonstrated
improvement in axial
disease symptoms1,2
Change from baseline in BASDAI
in patients with PsA with
imaging-confirmed sacroiliitis
Change from baseline in BASDAI
in patients with PsA with
imaging-confirmed sacroiliitis
Post hoc analysis: pooled data from DISCOVER 1 and DISCOVER 2: LS mean change from baseline in BASDAI at Week 24 and Week 52*†‡
Data from DISCOVER 2 only: LS mean change from baseline in BASDAI at Week 100
Post hoc analysis: pooled data from DISCOVER 1 and DISCOVER 2: LS mean change from baseline in BASDAI at Week 24 and Week 52*†‡
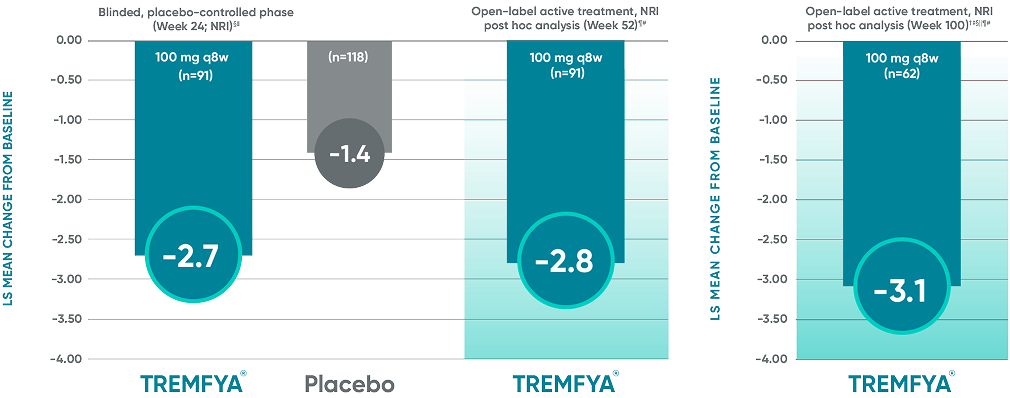
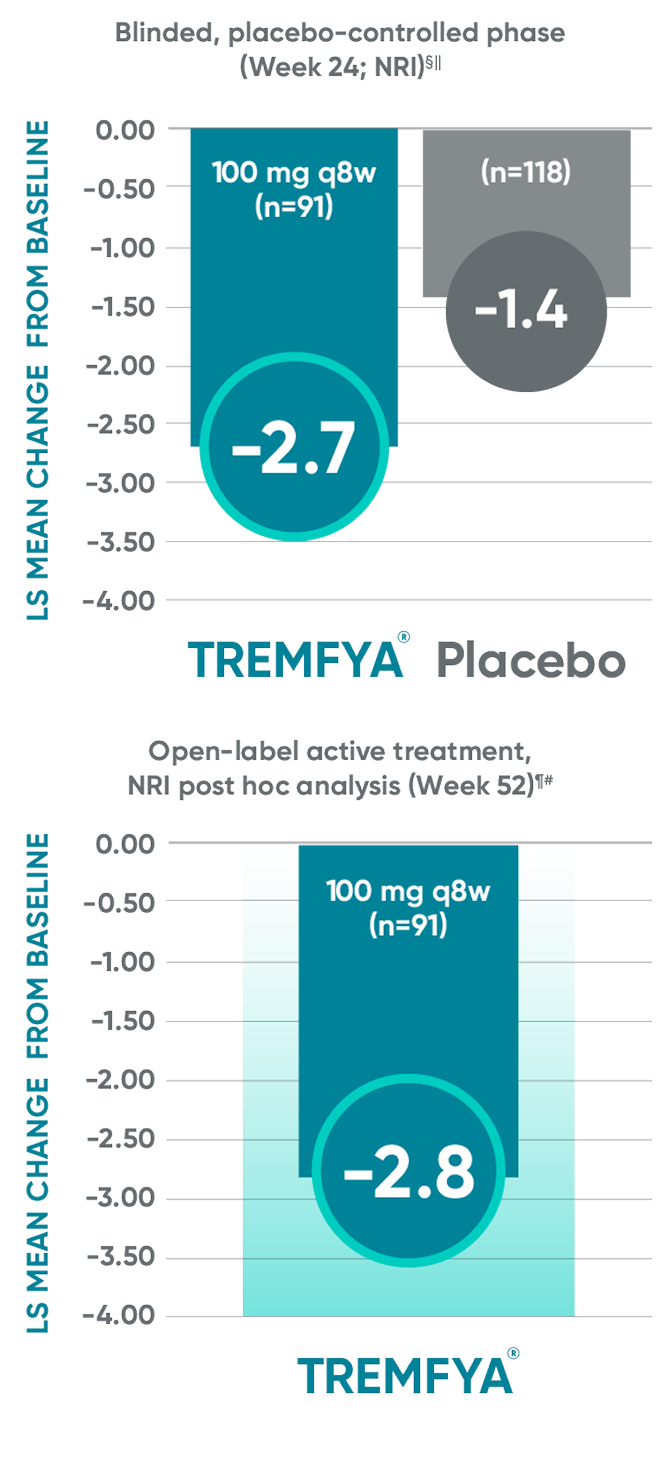
Data from DISCOVER 2 only:
LS mean change from baseline in BASDAI at Week 100
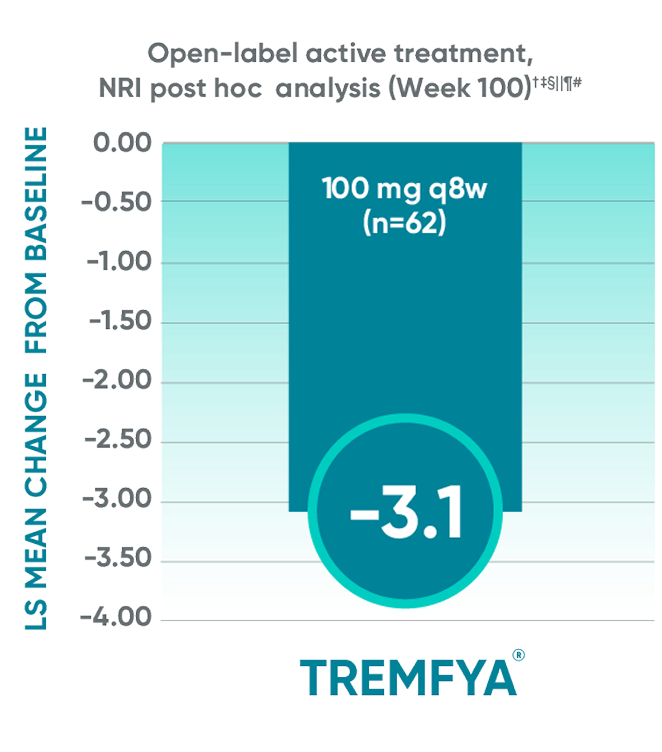
The BASDAI endpoints were not adjusted
for multiplicity. Therefore, statistical significance has not been established.
BASDAI=Bath Ankylosing Spondylitis Disease Activity Index; LS=least squares; NRI=nonresponder imputation; PsA=psoriatic arthritis; q8w=every 8 weeks.
*Included patients with sacroiliitis at baseline who
had either documented imaging confirmation of sacroiliitis in the past or pelvic X-ray confirmation of sacroiliitis at screening based on investigators’ judgment of presence/absence of sacroiliitis.
†BASDAI is a subject self-assessment of axial
disease, which asks patients to rate their symptoms
on a scale of 0 to 10, with higher scores indicating greater disease severity.
‡Through Week 24, patients were considered to have no improvement (change=0) after meeting
treatment failure criteria (see study designs). After Week 24, treatment failure rules were not applied.
§LS mean change for BASDAI scores was calculated using Mixed-Effect Repeated Measures.
||After subjects discontinued study agent due to any reason, the change from baseline, if missing, was considered to have no improvement (change=0).
¶After Week 24, patients and doctors knew that all patients were on TREMFYA® (open label with a
blinded dosing interval), which may have affected the results.
#The prespecified as-observed analyses at Week 52 and Week 100 are not shown.
BASDAI50 response in patients with imaging-confirmed sacroiliitis1,2
BASDAI50 response in patients with imaging-confirmed sacroiliitis1,2
Post hoc analysis: pooled data from DISCOVER 1 and DISCOVER 2: BASDAI50 at Week 24 and Week 52*†‡§||¶#
Post hoc analysis: data from DISCOVER 2 only: BASDAI50 at Week 100¶#
Post hoc analysis pooled data from DISCOVER 1 and DISCOVER 2: BASDAI at Week 24 and Week 52*†‡§||¶#
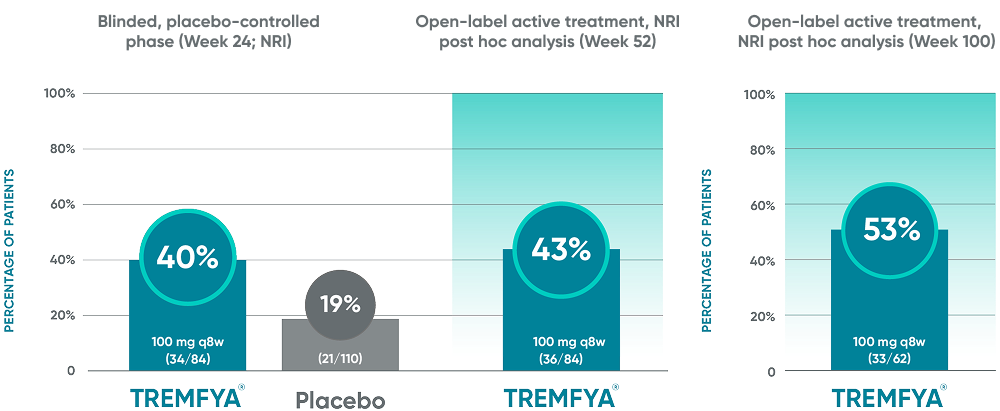
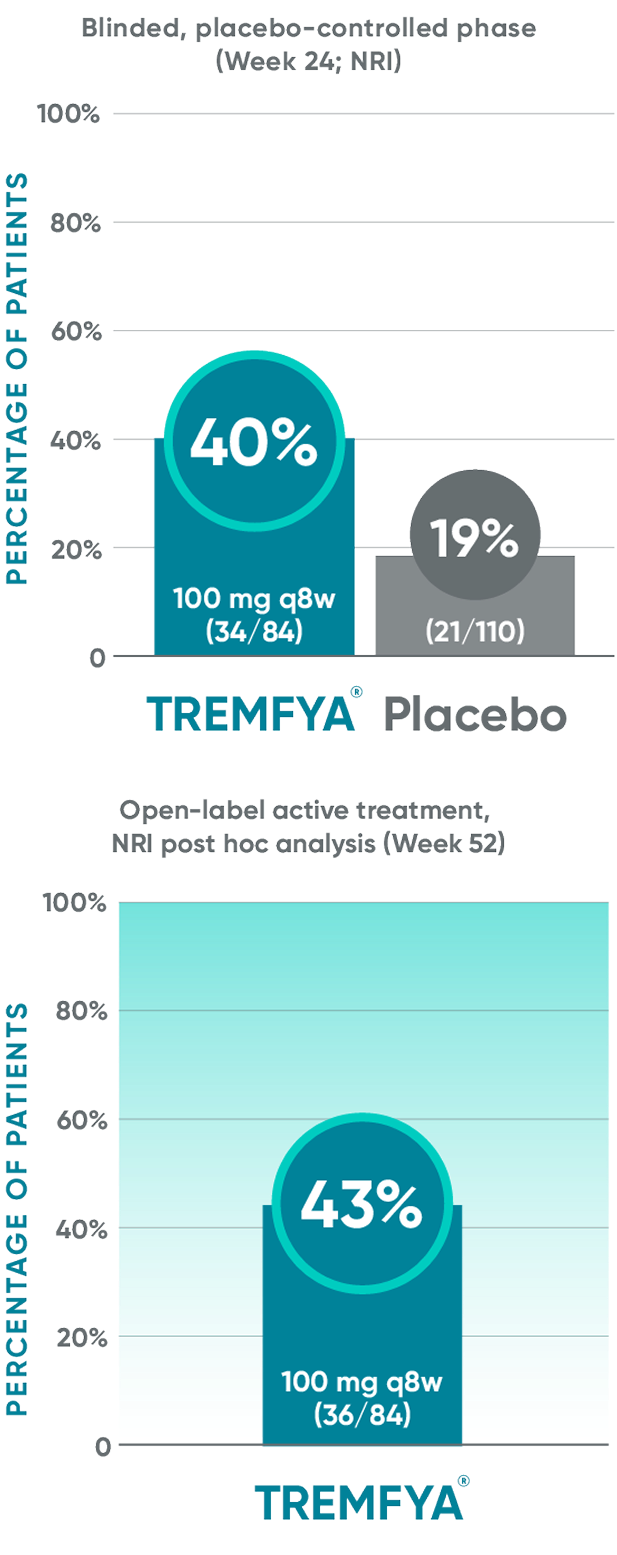
Post hoc analysis: data from DISCOVER 2 only: BASDAI50 at
Week 100¶#
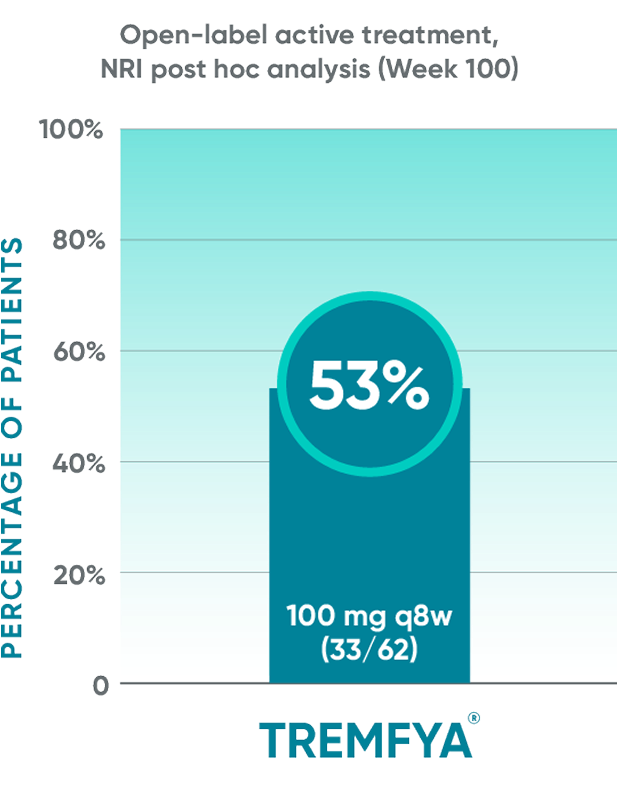
These endpoints were not adjusted for multiplicity. Therefore, statistical
significance has not been established.
*Included patients with sacroiliitis at baseline who
had either documented imaging confirmation of sacroiliitis in the past or pelvic X-ray confirmation of sacroiliitis at screening based on investigators’ judgment of presence/absence of sacroiliitis.
†BASDAI is a subject self-assessment of axial
disease, which asks patients to rate their symptoms
on a scale of 0 to 10, with higher scores indicating greater disease severity.
‡After Week 24, patients and doctors knew that all patients were on TREMFYA® (open label with a blinded dosing interval), which may have affected the results.
§The prespecified as-observed analyses at Weeks 52 and 100 are not shown.
||Patients with BASDAI >0 at baseline.
¶Patients with missing data were considered nonresponders.
#Through Week 24, patients were considered to be nonresponders after meeting treatment failure criteria (see study designs). After Week 24, treatment failure rules were not applied.
‡After Week 24, patients and doctors knew that all patients were on TREMFYA® (open label with a blinded dosing interval), which may have affected the results.
§The prespecified as-observed analyses at Weeks 52 and 100 are not shown.
IIPatients with BASDAI >0 at baseline.
¶Patients with missing data were considered nonresponders.
#Through Week 24, patients were considered to be nonresponders after meeting treatment failure criteria (see study designs). After Week 24, treatment failure rules were not applied.
Mean change from baseline in the BASDAI components at Week 24 in patients with imaging-confirmed sacroiliitis1
Post hoc analysis pooled data from DISCOVER 1 and DISCOVER 2:
BASDAI component scores at Week 24*†‡
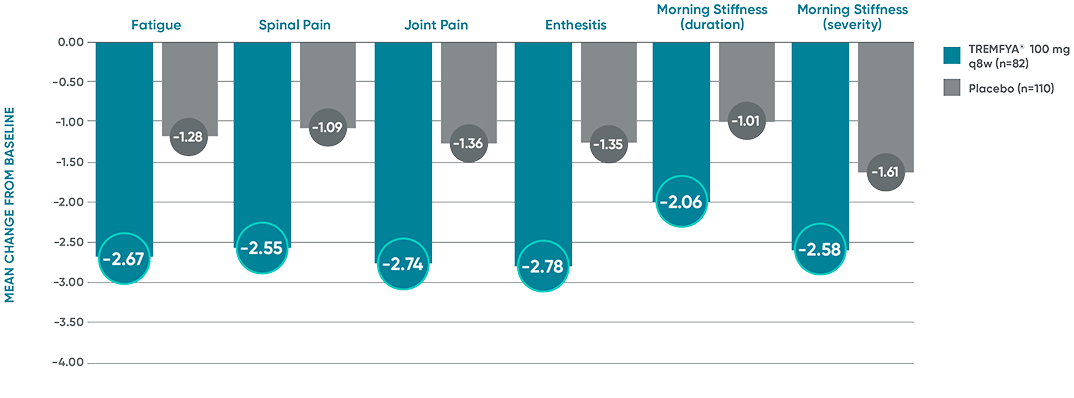
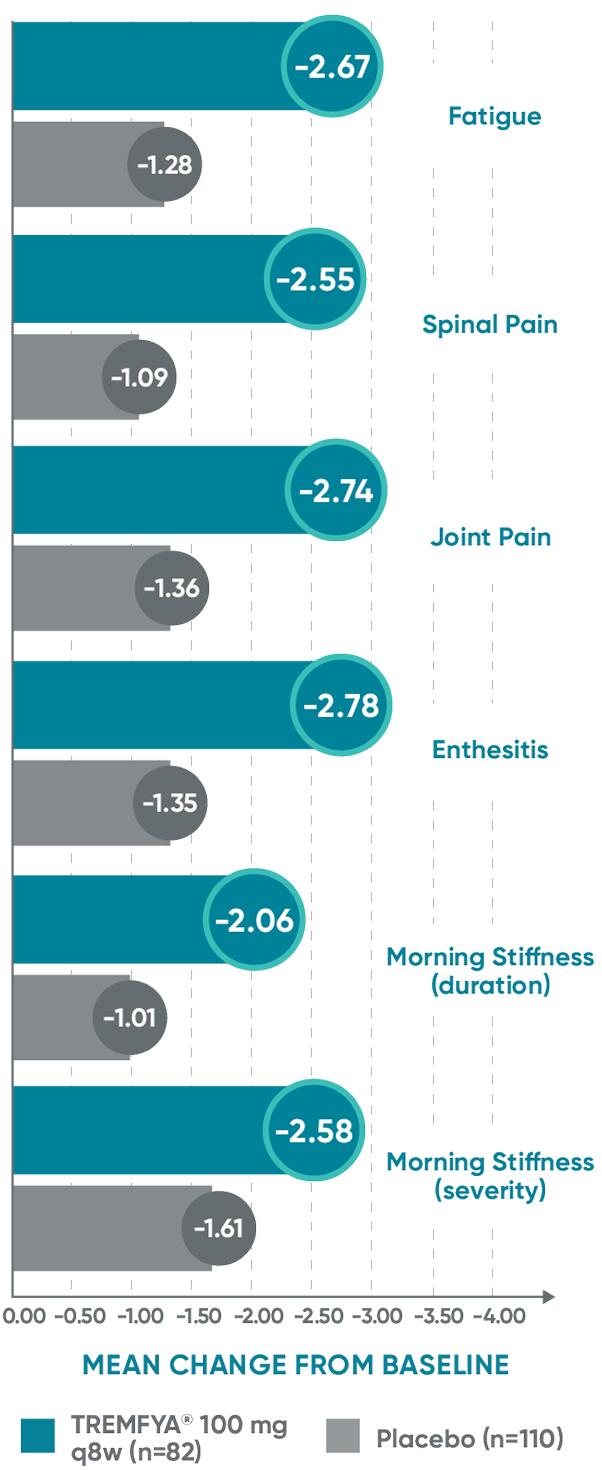
The BASDAI endpoints were not adjusted for multiplicity. Therefore, statistical
significance has not been established.
*Included patients with sacroiliitis at baseline who had either documented imaging confirmation of sacroiliitis in the past or pelvic X-ray confirmation of sacroiliitis at screening based on investigators’ judgment of presence/absence of sacroiliitis.
†BASDAI is a subject self-assessment of axial disease, which asks patients to rate their symptoms on a scale of 0 to 10, with higher scores indicating greater disease severity.
‡Through Week 24, patients were considered to have no improvement (change=0) after meeting treatment failure criteria (see study designs). After Week 24, treatment failure rules were not applied.
References: 1. Data on file. Janssen Biotech, Inc. 2. Helliwell PS, Gladman DD, Poddubnyy D, et al. Efficacy of guselkumab, a monoclonal antibody that specifically binds to the p19-subunit of IL-23, on endpoints related to axial involvement in patients with active PsA with imaging-confirmed sacroiliitis: week-24 results from two phase 3, randomized, double-blind, placebo-controlled studies. Poster presented at: European Alliance of Associations for Rheumatology (EULAR) Congress; Virtual; June 3-6, 2020.
Lasting skin clearance at 2 years
Treatment with TREMFYA® (guselkumab) resulted in an improvement in the skin manifestations of psoriasis in patients with ≥3% BSA of psoriatic involvement and IGA score ≥2 at baseline1-5*†‡
DISCOVER 2: IGA 0/1 response rates with a ≥2-grade improvement at
Weeks 24, 52, and 100†§||¶#
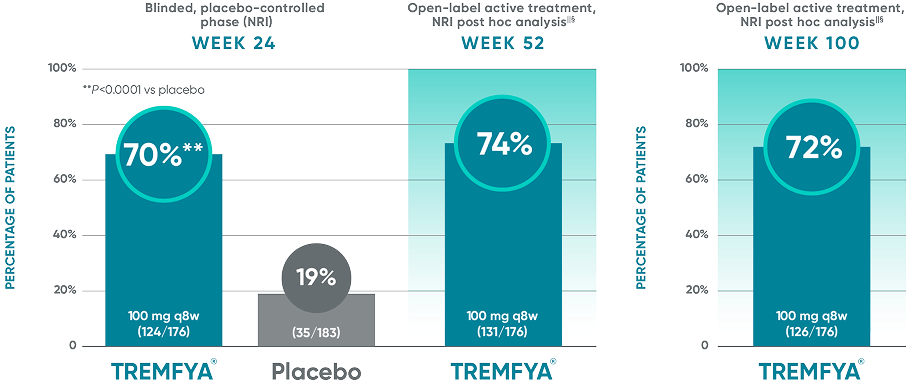
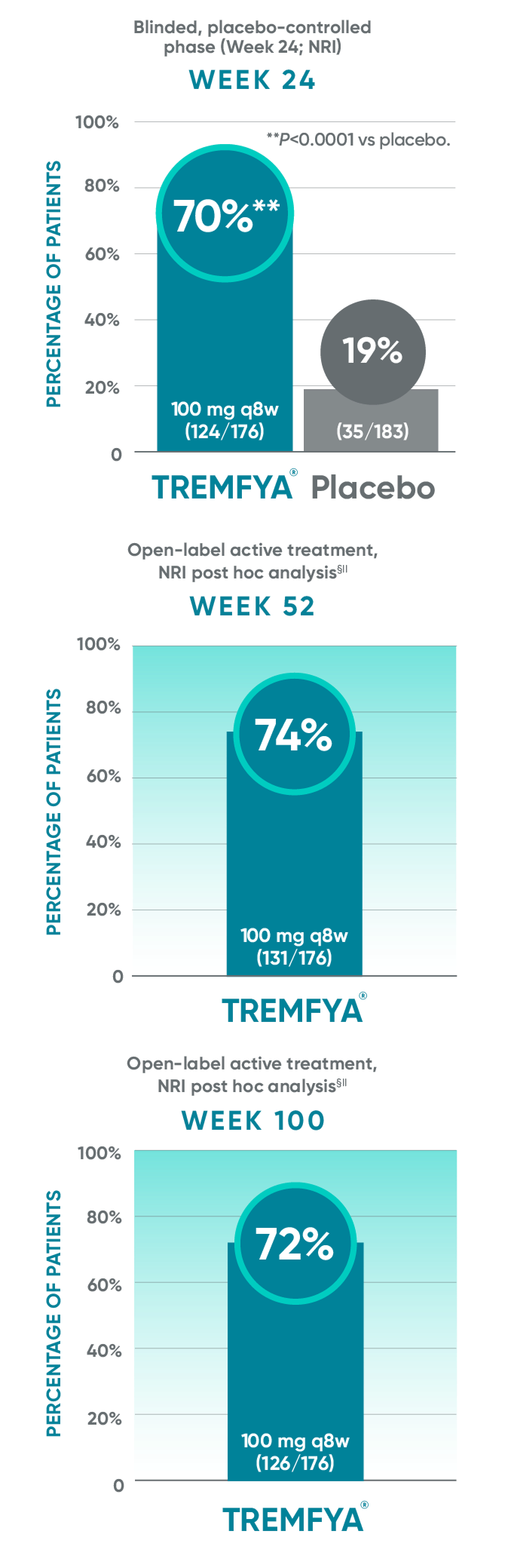
In DISCOVER 1
Week 24: 57% of patients receiving TREMFYA® q8w (47/82) had an IGA score of 0 (cleared) or 1 (minimal) and ≥2-grade reduction from baseline vs 15% of patients receiving placebo (12/78) (P<0.0001).1,2,6ǁ¶
Week 52 (NRI post hoc analysis): 63% of patients receiving TREMFYA® q8w (52/82) had an IGA score of 0 (cleared) or 1 (minimal) and ≥2-grade reduction from baseline.1,7§ǁ¶††
BSA=body surface area; IGA=Investigator’s Global Assessment; NRI=nonresponder imputation; q8w=every 8 weeks.
*The same patients may not have responded at each time point.
†IGA 0/1=proportion of patients who achieved an IGA score of cleared (0) or minimal (1) using a 5-point scale where psoriatic lesions are graded by the investigator for induration, erythema, and scaling on a scale of 0 to 4: cleared, except for residual discoloration (0), minimal (1), mild (2), moderate (3), or severe (4).
‡Year 2 represents Week 100.
§After Week 24, patients and doctors knew that all patients were on TREMFYA® (open label with a blinded dosing interval), which may have affected the results.
||Through Week 24, patients were considered to be nonresponders after meeting treatment failure criteria (see study designs). After Week 24, treatment failure rules were not applied.
¶Patients with missing data were considered nonresponders.
#The DISCOVER 2 prespecified as-observed analysis from Weeks 24 to 100 is not shown.
††The DISCOVER 1 prespecified as-observed analysis from Weeks 24 to 52 is not shown.
PASI 90 response rates at 2 years with TREMFYA®1-5*
Treatment with TREMFYA® resulted in an improvement in the skin manifestations
of psoriasis in subjects with PsA
DISCOVER 2: PASI 90 response rates at Week 24, Week 52, and Week 100 in patients
with ≥3% of psoriatic involvement and IGA score ≥2 at baseline†‡
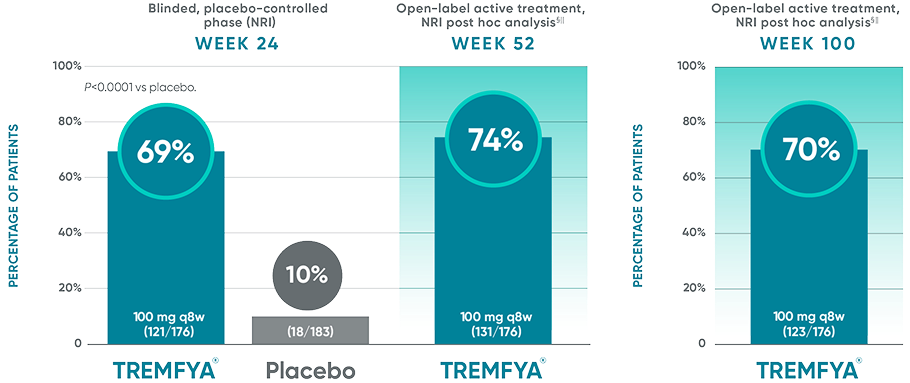
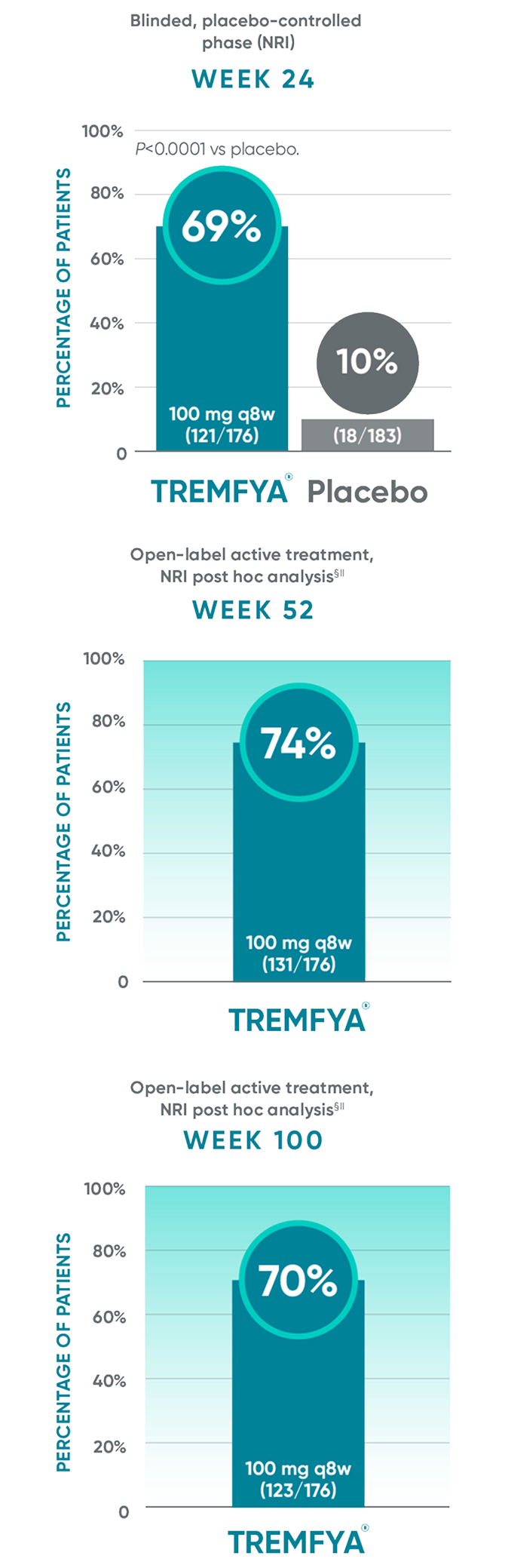
PASI 90 endpoints were not adjusted for multiplicity. Therefore, statistical significance has not been established.
*The same patients may not have responded at each time point.
In DISCOVER 1
Week 24: 50% of patients receiving TREMFYA® q8w (41/82) achieved a PASI 90 response vs 12% of patients receiving placebo (9/78)1,2,6†‡
Week 52 (NRI post hoc analysis): 61% of patients receiving TREMFYA® q8w (50/82) achieved a PASI 90 response1,7*†‡§¶
IGA=Investigator's Global Assessment; PASI=Psoriasis Area and Severity Index.
†Through Week 24, patients were considered to be nonresponders after meeting treatment failure criteria (see study designs). After Week 24, treatment failure rules were not applied.
‡Patients with missing data were considered nonresponders.
§After Week 24, patients and doctors knew that all patients were on TREMFYA® (open label with a blinded dosing interval), which may have affected the results.
IIThe DISCOVER 2 prespecified as-observed analysis from Weeks 24 to 100 is not shown.
¶The DISCOVER 1 prespecified as-observed analysis from Weeks 24 to 52 is not shown.
References: 1. TREMFYA® (guselkumab) [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc. 2. Data on file. Janssen Biotech, Inc. 3. Mease PJ, Rahman P, Gottlieb AB, et al. Guselkumab in biologic-naïve patients with active psoriatic arthritis (DISCOVER 2): a double-blind, randomized, placebo-controlled phase 3 trial. Lancet. 2020;395(10230):1126-1136. 4. McInnes IB, Rahman P, Gottlieb AB, et al. Long-term efficacy and safety of guselkumab, a monoclonal antibody specific to the p19 subunit of interleukin-23, through two years: results from a phase III, randomized, double-blind, placebo-controlled study conducted in biologic-naïve patients with active psoriatic arthritis. Arthritis Rheumatol. 2022;74(3):475-485. 5. McInnes IB, Rahman P, Gottlieb AB, et al. Efficacy and safety of guselkumab, an interleukin-23p19-specific monoclonal antibody, through one year in biologic-naïve patients with psoriatic arthritis. Arthritis Rheumatol. 2021;73(4):604-616. 6. Deodhar A, Helliwell PS, Boehncke W-H, et al. Guselkumab in patients with active psoriatic arthritis who were biologic-naïve or had previously received TNFα inhibitor treatment (DISCOVER 1): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395(10230):1115-1125. 7. Ritchlin CT, Helliwell PS, Boehncke W-H, et al. Guselkumab, an inhibitor of the IL-23-p19 subunit, provides sustained improvement in signs and symptoms of active psoriatic arthritis: 1 year results from a phase III randomised study of patients who were biologic-naïve or TNFα inhibitor-experienced. RMD Open. 2021;7(1):e001457.
IN ADULT PATIENTS WITH MODERATE TO SEVERE PLAQUE PSORIASIS (PsO)
VOYAGE 1 & VOYAGE 2 co-primary endpoints at Week 161,2

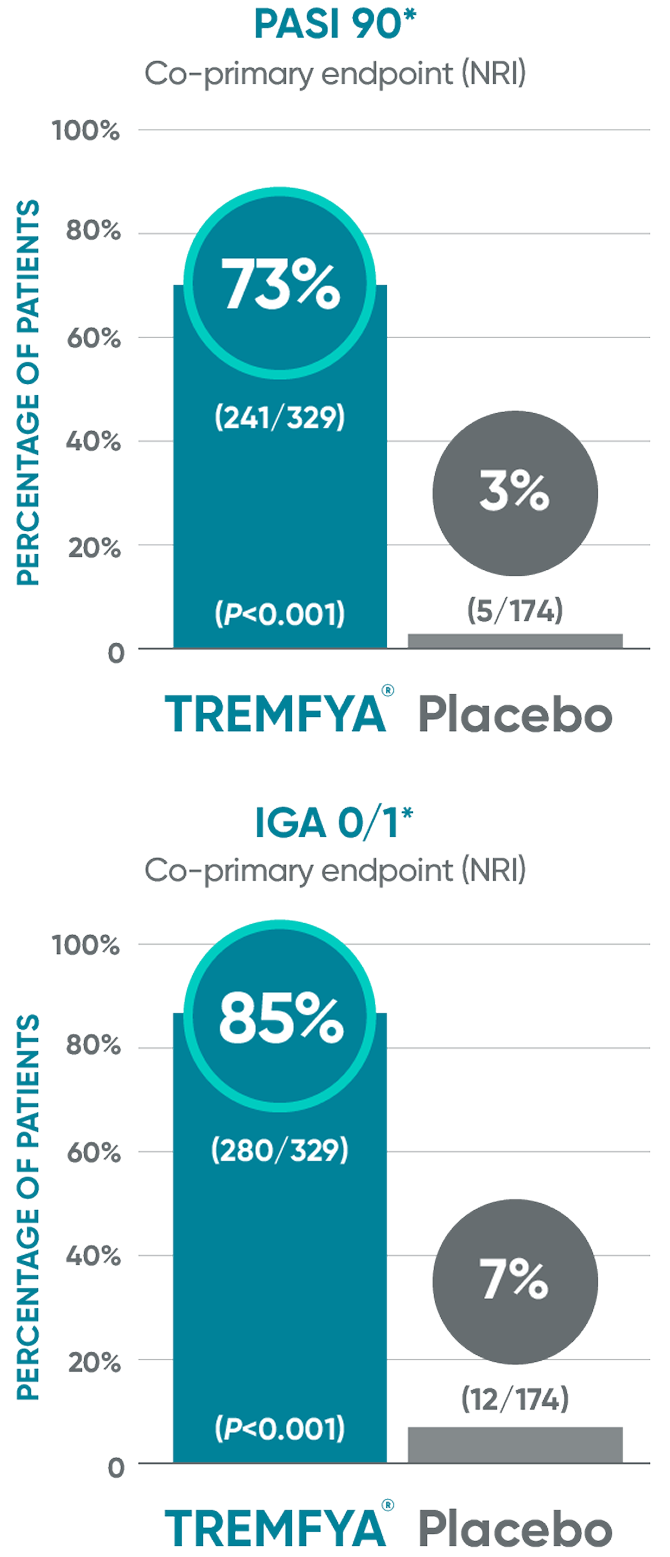
In VOYAGE 2 at Week 16 (co-primary endpoints, NRI)1,3:
IGA 0/1=Investigator's Global Assessment. IGA score of cleared (0) or minimal (1) using a 5-point scale of overall disease severity; NRI=nonresponder imputation; PASI=Psoriasis Area and Severity Index.
*NRI methods were used for analysis.
Difficult-to-treat
patient types: Nail
data
VOYAGE 1: Prespecified other secondary analysis—mean percentage
improvement from baseline NAPSI (target nail, NRI)2*†‡§
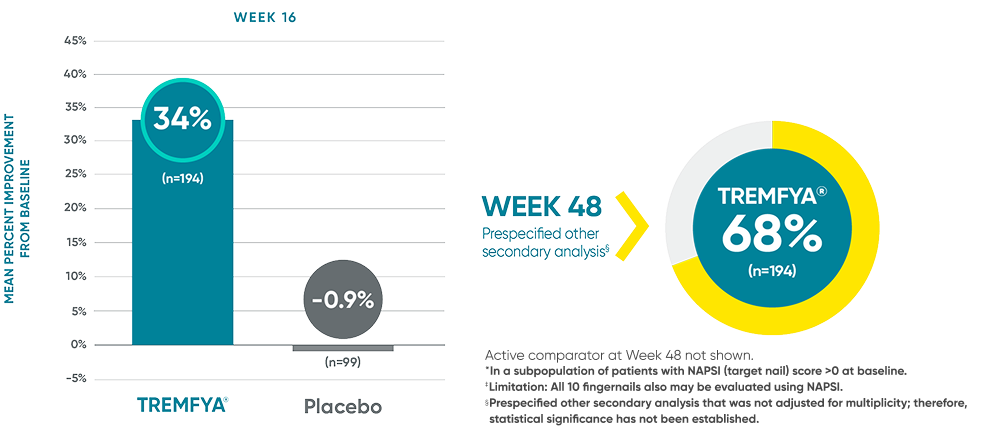
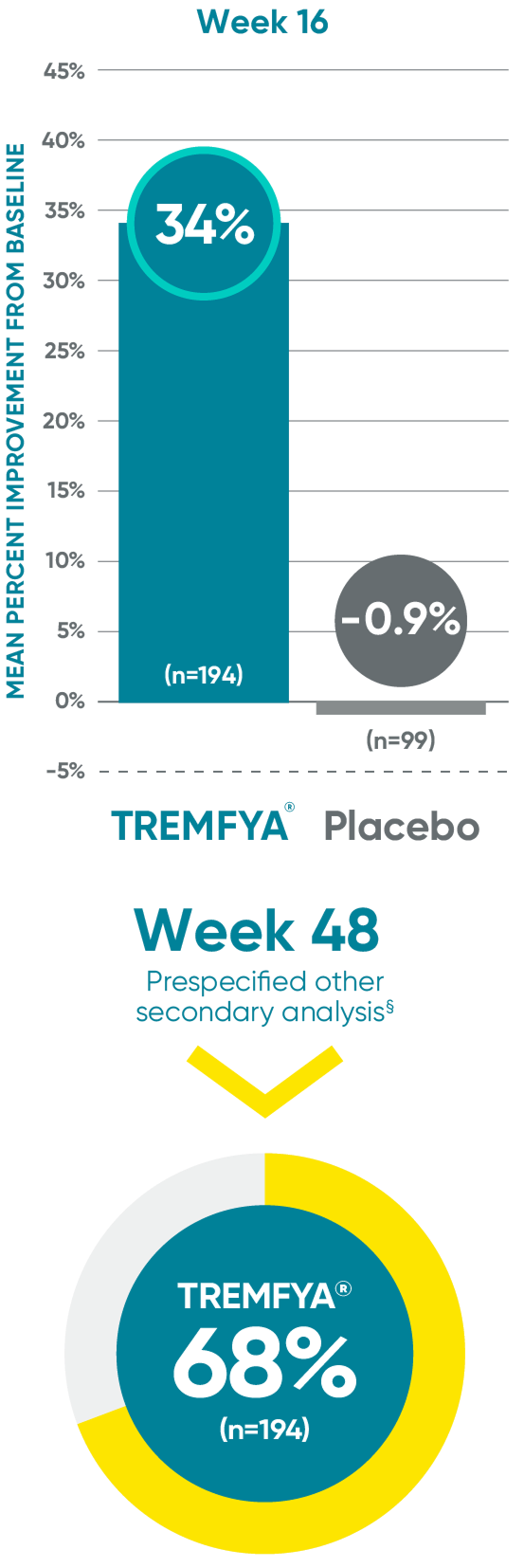
Active comparator at Week 48 not shown.
*In a subpopulation of patients with NAPSI (target nail) score >0 at baseline.
‡Limitation: All 10 fingernails also may be evaluated using NAPSI.
§Prespecified other secondary analysis that was not adjusted for multiplicity; therefore, statistical significance has not been established.
VOYAGE 2: Prespecified other secondary analysis—mean percent improvement from baseline NAPSI (target nail, NRI)3*†‡§
40% (n=280) of patients treated with TREMFYA® achieved mean percent improvement from baseline at Week 16 vs 2% (n=140) for placebo.
NAPSI=Nail Psoriasis Severity Index.
†Fingernail psoriasis was assessed using NAPSI, in which the nail most affected by psoriasis (target nail) is divided into quadrants and graded for psoriasis of the nail matrix (pitting, leukonychia, red spots in the lunula, and nail plate crumbling) and nail bed (onycholysis, splinter hemorrhages, oil drop discoloration, and nail bed hyperkeratosis) on a scale of 0 to 4 for a total score ranging from 0 to 8; a higher score indicates more severe disease.
References: 1. TREMFYA® (guselkumab) [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc. 2. Blauvelt A, Papp KA, Griffiths CEM, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76(3):405-417. 3. Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76(3):418-431.