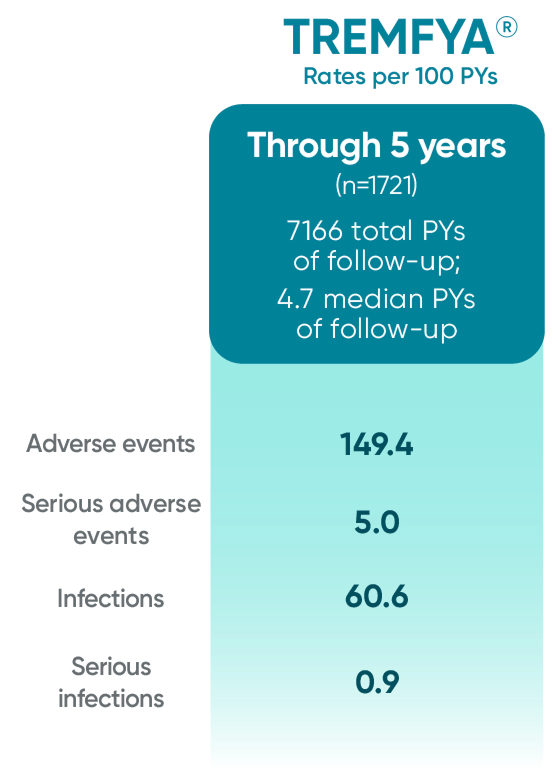
TREMFYA® (guselkumab) proven safety profile through
2 years*
Adverse events reported in the placebo-controlled phase through
Week 24 combined across DISCOVER 1 and DISCOVER 21
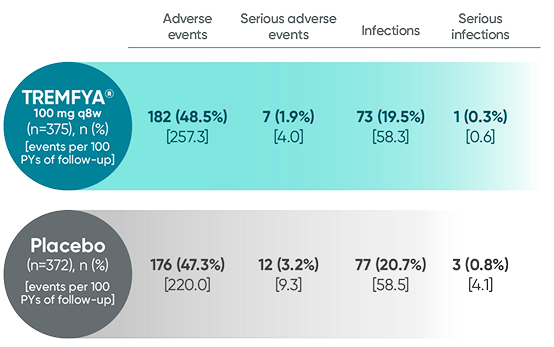
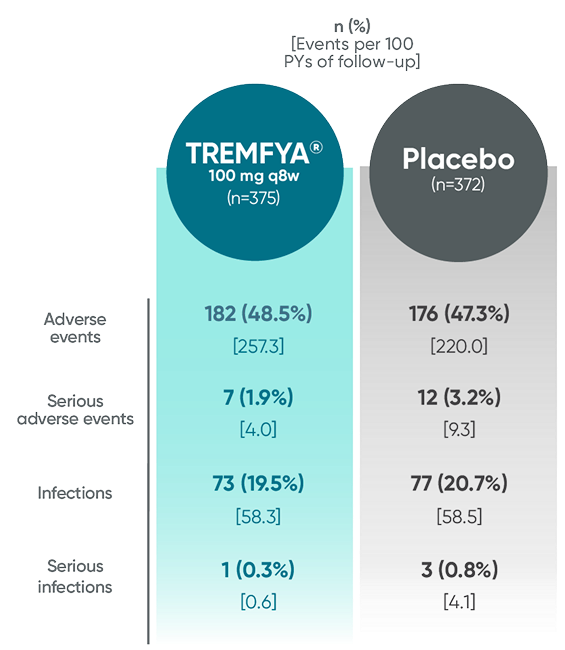
In the 24-week, placebo-controlled period of the combined DISCOVER 1 and DISCOVER 2 clinical trials1:
- The overall safety profile observed in patients with PsA treated with TREMFYA® is generally consistent with the profile in patients with plaque PsO, with the addition of bronchitis (occurred in 1.6% and 1.1% of patients in the TREMFYA® q8w group and placebo group, respectively) and neutrophil count decreased (occurred in 0.3% and 0% of patients in the TREMFYA® q8w group and placebo group, respectively)1:
- The majority of events of neutrophil count decreased were mild, transient, not associated with infection, and did not lead to discontinuation
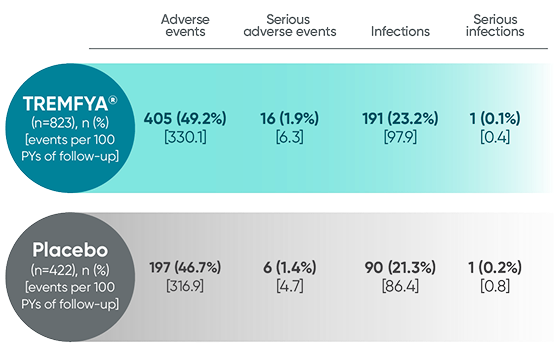
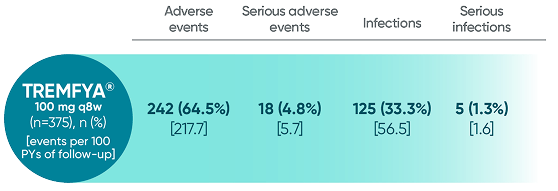
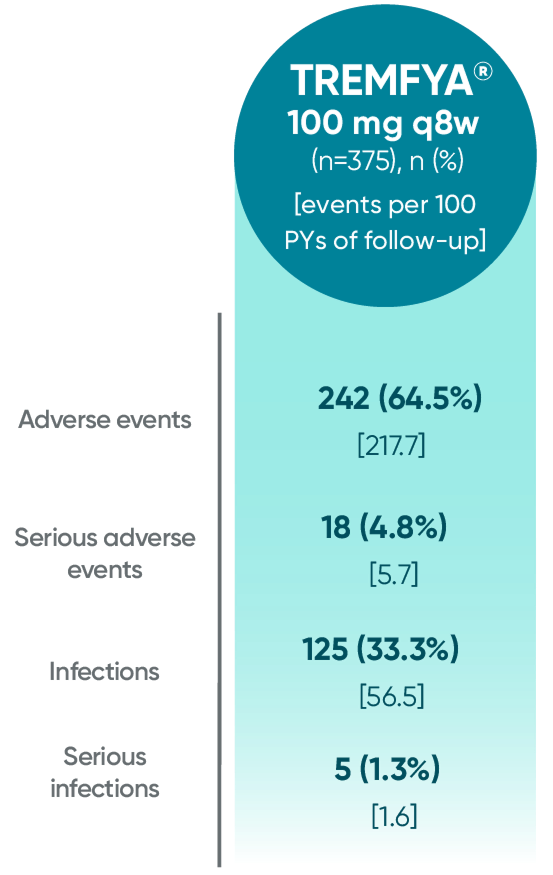
Adverse events reported through 1 year combined across
DISCOVER 1 and DISCOVER 21†
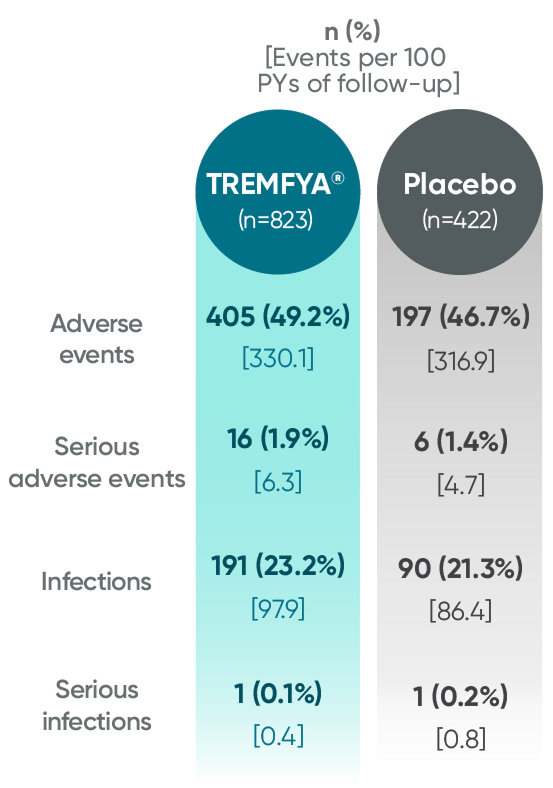
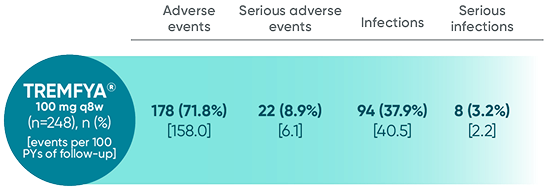
Adverse events reported through end of study (Week 112)
in DISCOVER 2 only1,2
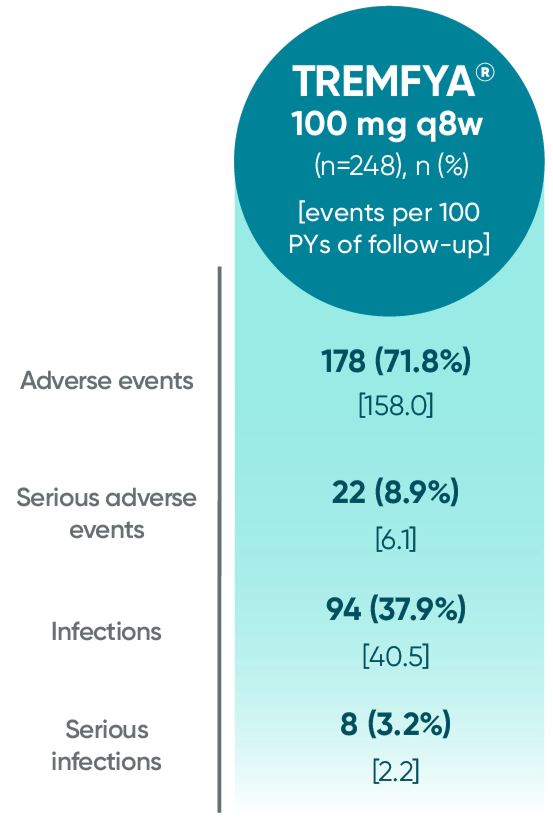
PYs=patient-years; q8w=every 8 weeks.
*Through Week 112 in DISCOVER 2.
†1 year is defined as 60 weeks (through end of study) in DISCOVER 1 and 52 weeks in DISCOVER 2.
References: 1. Data on file. Janssen Biotech, Inc. 2. McInnes IB, Rahman P, Gottlieb AB, et al. Long-term efficacy and safety of guselkumab, a monoclonal antibody specific to the p19 subunit of interleukin-23, through two years: results from a phase III, randomized, double-blind, placebo-controlled study conducted in biologic-naïve patients with active psoriatic arthritis. Arthritis Rheumatol. 2022;74(3):475-485.