Induction Results in SC (GRAVITI) and IV (GALAXI)
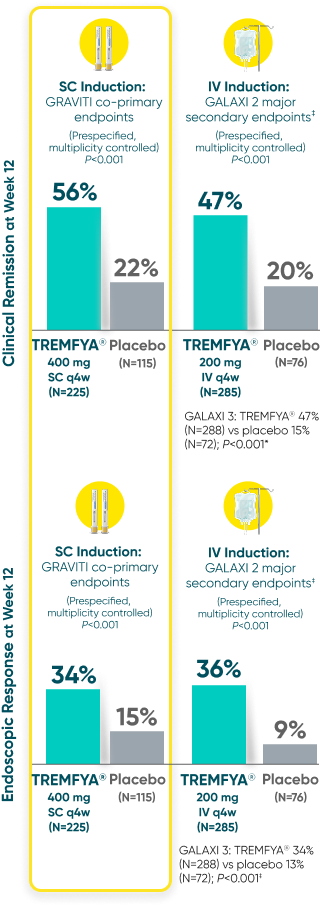
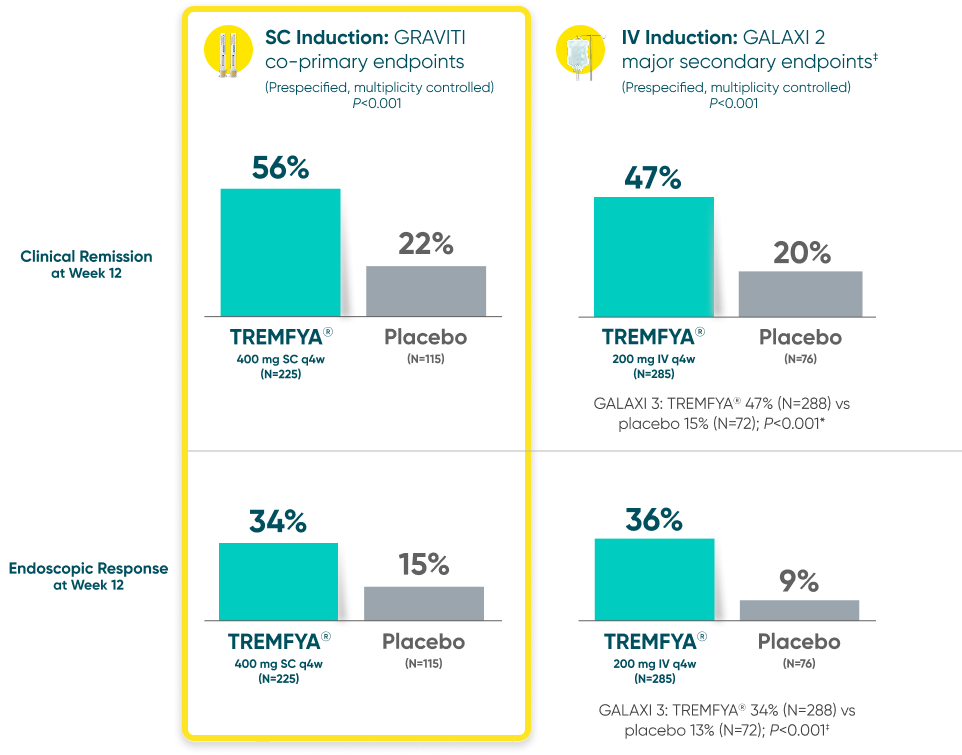
GRAVITI and GALAXI are separate studies with different endpoint assessments. Cross-trial comparisons are not to be made.
TREMFYA® showed early separation from placebo in clinical
response by Week 4 for both SC and IV induction2


GRAVITI and GALAXI are separate studies with different endpoint assessments. Cross-trial comparisons are not to be made.

DATA LIMITATION:
For GRAVITI, clinical response at Weeks 4 and 8 were prespecified but not adjusted for multiplicity. For both GALAXI studies, clinical response at Week 4 was adjusted for multiplicity (with nominal P-value), while data for Weeks 8 and 12 were not. No statistical significance can be made.
Clinical response: ≥100-point reduction from baseline in CDAI score1
CDAI=Crohn’s Disease Activity Index; IV=intravenous; q4w=every 4 weeks; SC=subcutaneous.
MAINTENANCE RESULTS IN GRAVITI (SC Induction)
With TREMFYA®, patients were in deep remission at 1 year1
Deep remission was defined as clinical remission and endoscopic remission at 1 year




DATA LIMITATION: Endoscopic remission at Week 48 was a prespecified exploratory endpoint not adjusted for multiplicity. No statistical significance can be made.2
*Clinical remission defined as CDAI score <150.1
†Endoscopic response was defined as >50% improvement from baseline in SES-CD score.1
‡Combined data for both TREMFYA® 100 mg SC q8w and 200 mg SC q4w dosing arms. Patients in both arms received the same induction dose of TREMFYA® 200 mg (IV infusion) at Weeks 0, 4, and 8.1
§Endoscopic remission was defined as SES-CD score ≤4 and ≥2-point reduction from baseline and no subscore >1 in any individual component.1
||Patients in both 200 mg SC q4w and 100 mg SC q8w groups also received 3 SC induction doses.
CDAI=Crohn’s Disease Activity Index; GI=gastrointestinal; IV=intravenous; q4w=every 4 weeks; q8w=every 8 weeks; SC=subcutaneous; SES-CD=Simple Endoscopic Score for Crohn’s Disease.
References: 1. TREMFYA® [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc. 2. Data on file. Janssen Biotech, Inc.




