~90% patient retention through 2 years with TREMFYA®1*
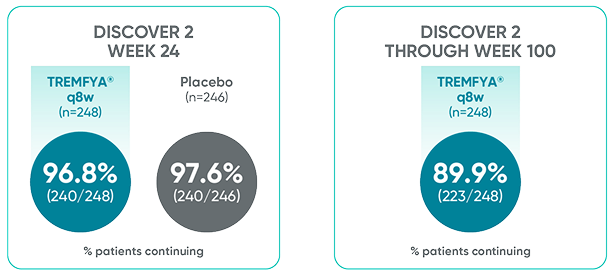
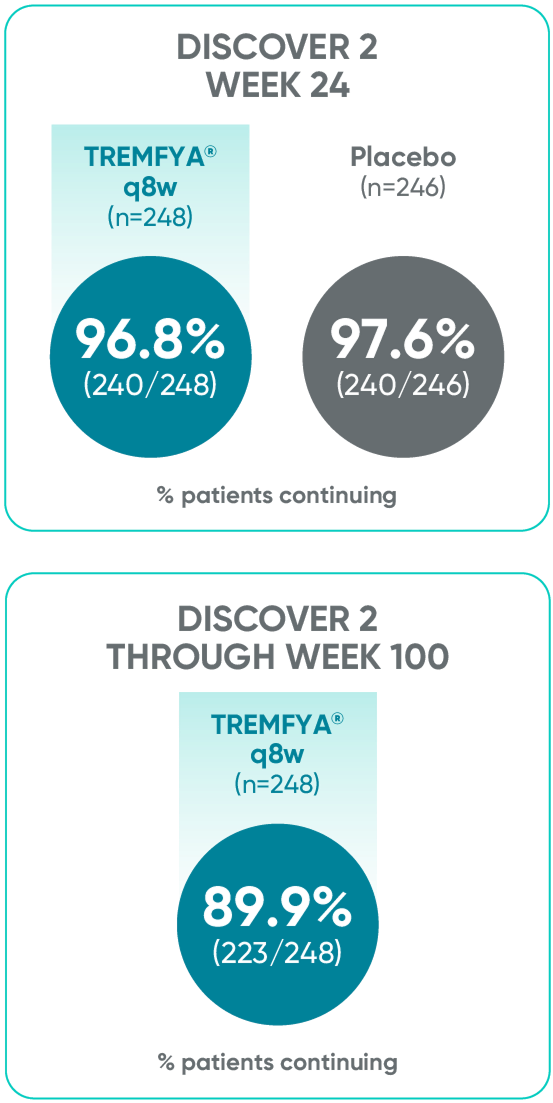
Patients continued therapy through Week 100 in DISCOVER 2
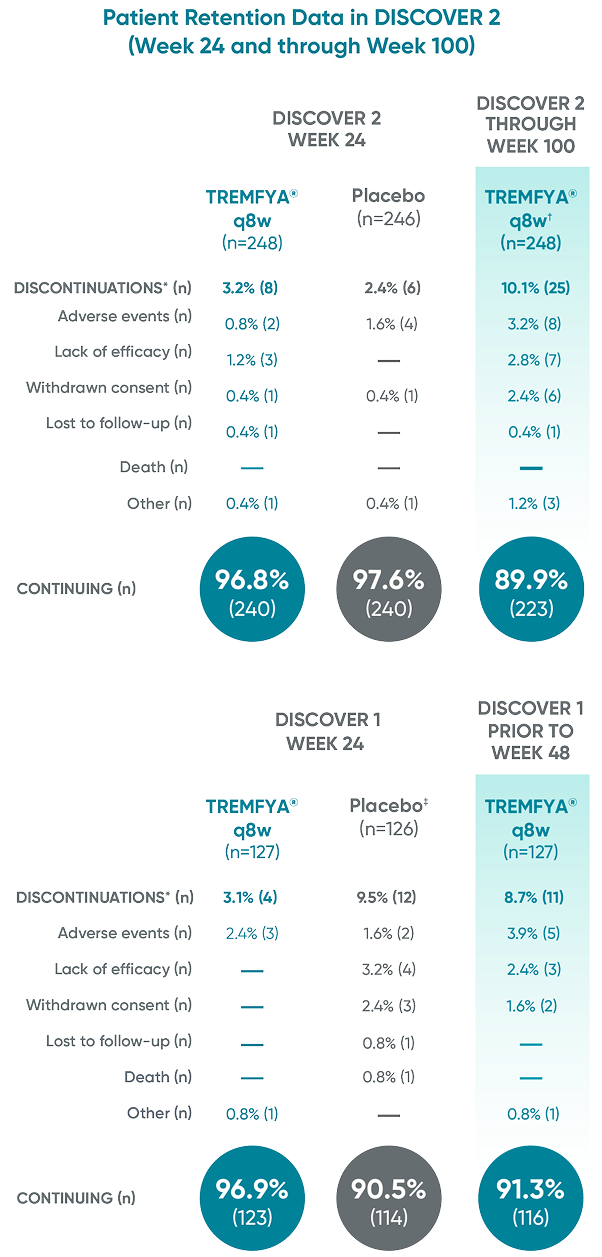
Patient Retention Data in DISCOVER 2 (Week 24 and through Week 100)
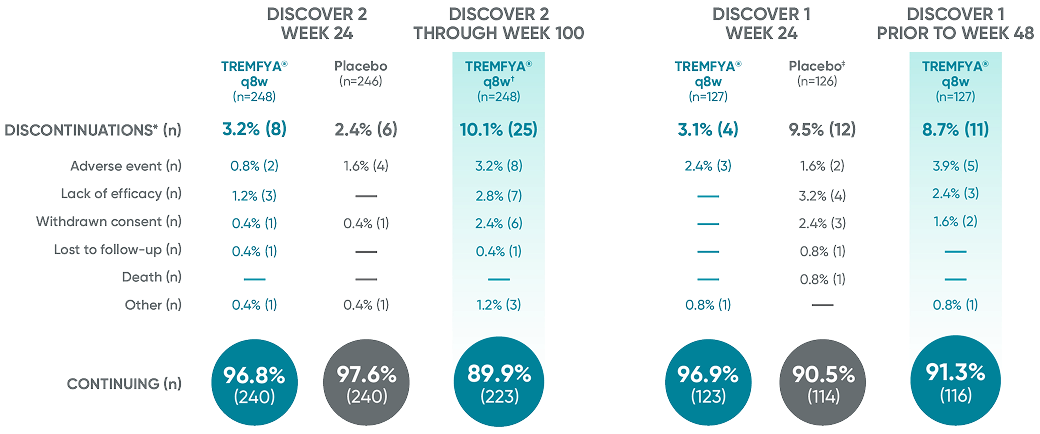
Reasons for discontinuation included: adverse event, lack of efficacy, withdrawn consent, lost
to follow-up, death, and other.
No conclusions regarding clinical efficacy or safety can be drawn.
q8w=every 8 weeks.
*Year 2 represents Week 100.
Patient retention data in DISCOVER 2 (Week 24 and through Week 100) and in DISCOVER 1
(Week 24 and prior to Week 48)1
No conclusions regarding clinical efficacy or safety can be drawn.
FDA=U.S. Food and Drug Administration; q4w=every 4 weeks.
*Discontinuations shown for patients who were randomized and received at least 1 study drug administration.
†248 patients in DISCOVER 2 were randomized to
TREMFYA® 100 mg q8w dosing regimen through Week 112; TREMFYA® q4w was
not an FDA-approved dosing regimen.
‡1 patient-initiated, protocol-prohibited
medication in the placebo group through Week 24.
Reference: 1. Data on file. Janssen Biotech, Inc.