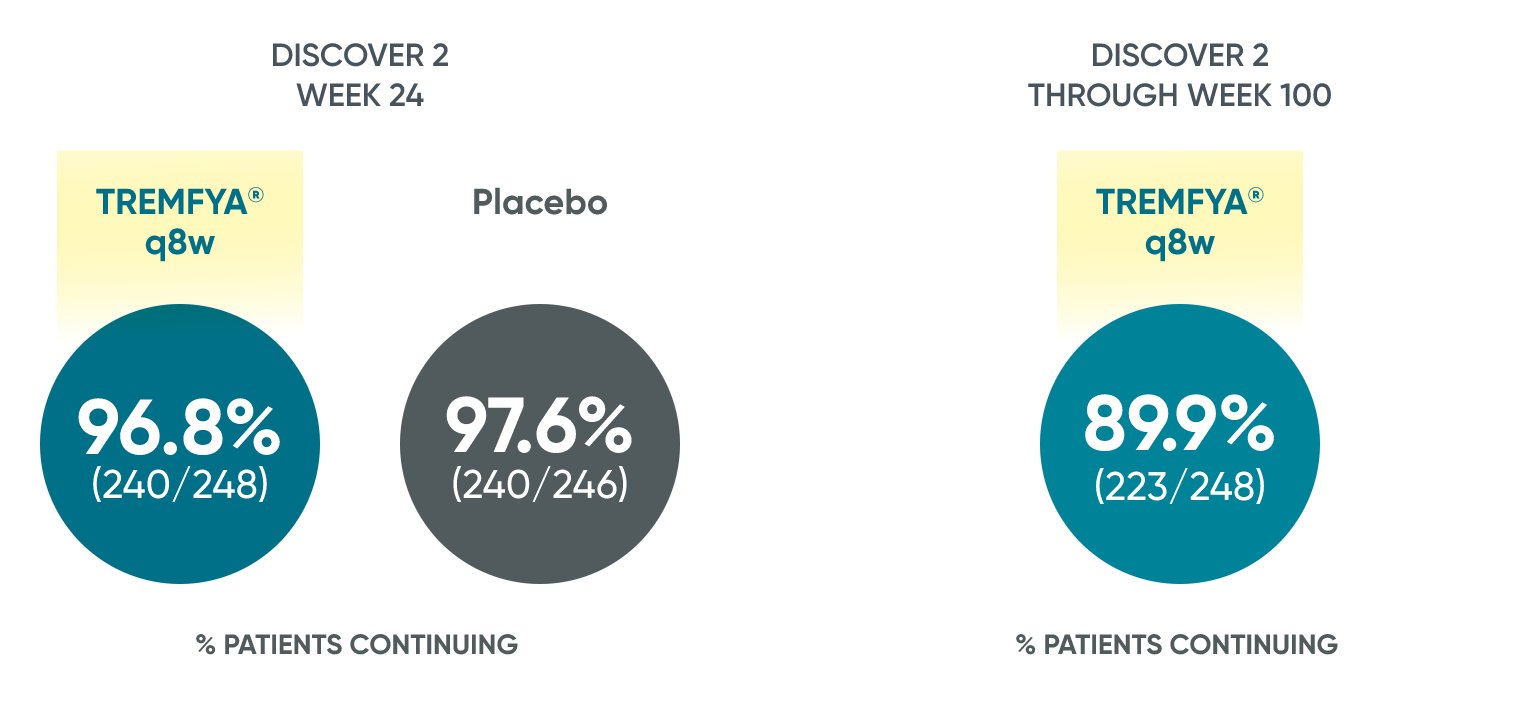
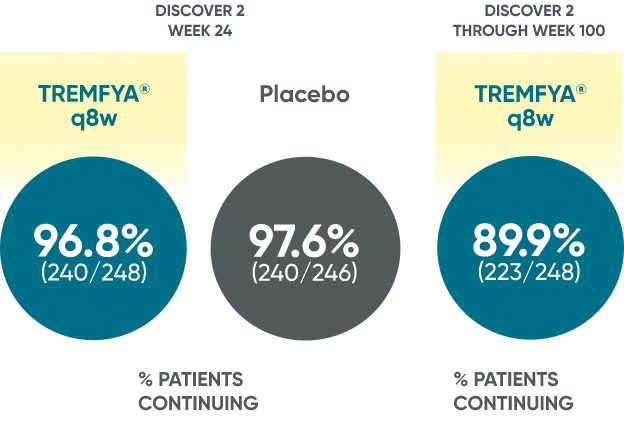
~90% patient retention through 2 years with TREMFYA®1*
Patients continued therapy through Week 100 in DISCOVER 2
Patient Retention Data in DISCOVER 2 (Week 24 and through Week 100)
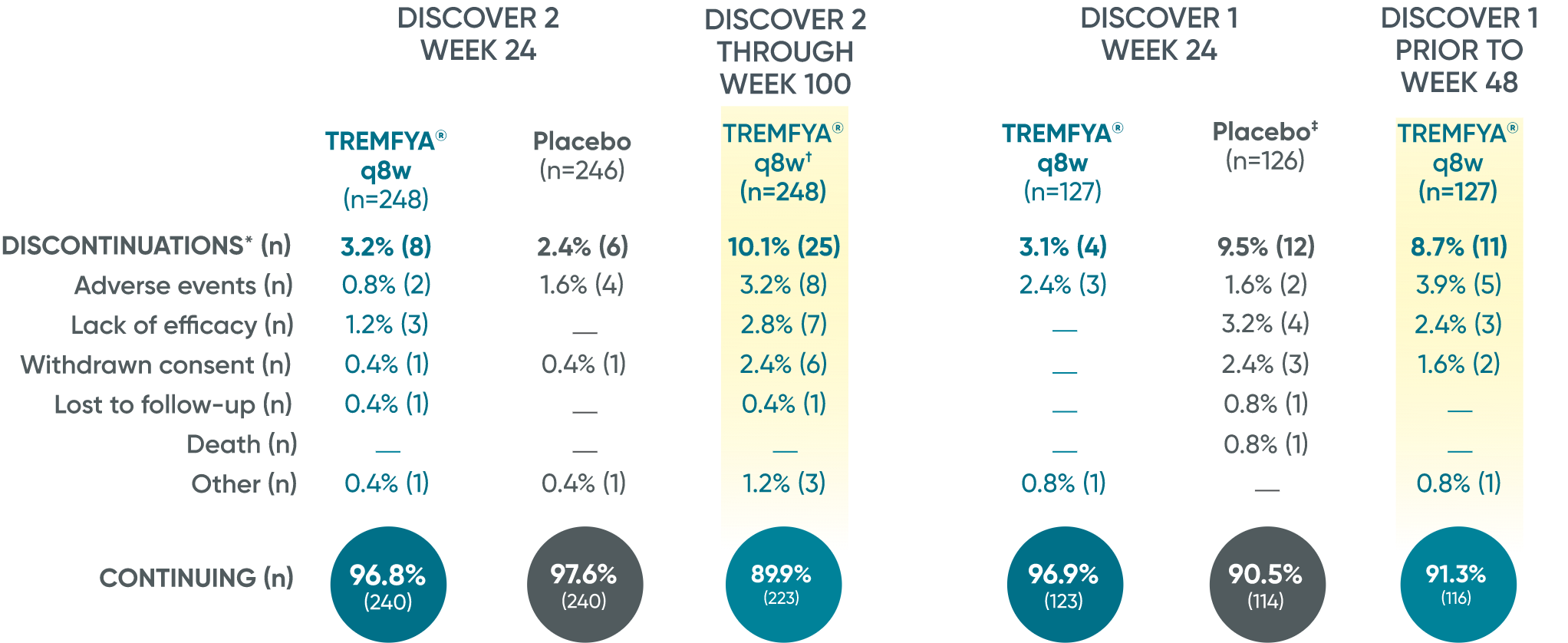
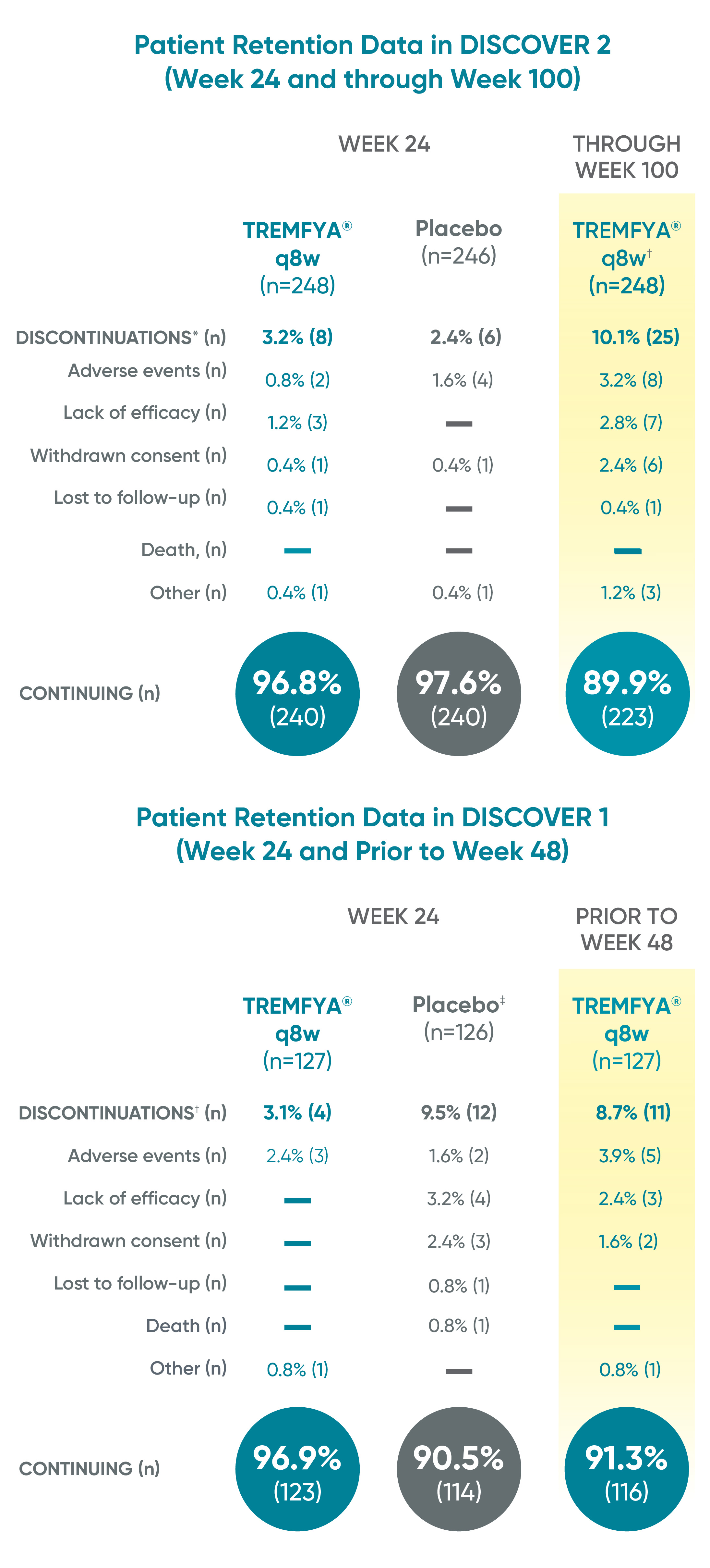
Reasons for discontinuation included: adverse event, lack of efficacy, withdrawn consent, lost to follow-up, death, and other.
No conclusions regarding clinical efficacy or safety can be drawn.
*Year 2 represents Week 100.
Patient retention data in DISCOVER 2 (Week 24 and through Week 100) and in DISCOVER 1 (Week 24 and prior to Week 48)1
No conclusions regarding clinical efficacy or safety can be drawn.
*Discontinuations shown for patients who were randomized and received at least 1 study drug administration.
†248 patients in DISCOVER 2 were randomized to TREMFYA® 100 mg q8w dosing regimen through Week 112; TREMFYA® q4w was not an FDA-approved dosing regimen.
‡1 patient-initiated, protocol-prohibited medication in the placebo group through Week 24.
Reference: 1. Data on file. Janssen Biotech, Inc.