TREMFYA® PEN
TREMFYA® comes in a single-dose prefilled pen containing one 200-mg dose. Each TREMFYA® PEN can be used only one time.
TREMFYA® offers one single subcutaneous (SC) pen injection for each maintenance dose1
200 mg/2 mL
TREMFYA® PEN


Not actual size.
TREMFYA® PEN
TREMFYA® comes in a single-dose prefilled pen containing one 200-mg dose. Each TREMFYA® PEN can be used only one time.
OR
Prefilled syringe

Not actual size.
Prefilled syringe
TREMFYA® comes as a single-dose prefilled syringe containing one 200-mg dose. Each TREMFYA® prefilled syringe can be used only one time.
Also in
100 mg/mL1
TREMFYA® PEN

Not actual size.
TREMFYA® PEN
TREMFYA® comes in a single-dose prefilled pen containing one 100-mg dose. Each TREMFYA® PEN can be used only one time.
OR
One-Press

Not actual size.
One-Press
TREMFYA® comes in a single-dose One-Press patient-controlled injector containing one 100-mg dose. Each One-Press injector can be used only one time.
OR
Prefilled syringe
Not actual size.
Prefilled syringe
TREMFYA® comes as a single-dose prefilled syringe containing one 100-mg dose. Each TREMFYA® prefilled syringe can be used only one time.
At induction, there is a 200-mg IV infusion administered over at least 1 hour at Week 0, Week 4, and Week 8.1
In maintenance, once you have determined the appropriate dose for your patient, you and your patient can choose which device may be best suited for them.
Use the lowest effective recommended dosage to maintain therapeutic response.
Please see the full Instructions for Use for the TREMFYA® PEN, One-Press patient-controlled injector, and prefilled syringe.

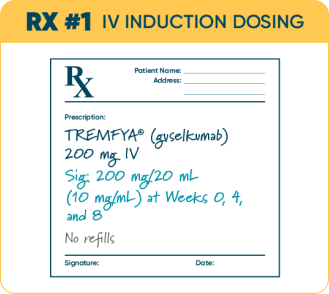
Write one Rx for TREMFYA® UC IV Induction (200 mg IV q4w).*
The recommended induction dosage of TREMFYA® is 200 mg administered by intravenous infusion over at least 1 hour at Week 0, Week 4, and Week 8.

Write one Rx for TREMFYA® UC SC Maintenance (200 mg SC q4w or 100 mg SC q8w).
The recommended maintenance dosage of TREMFYA® is:
OR
Use the lowest effective recommended dosage to maintain therapeutic response.
Please refer to the full Prescribing Information for the complete dosing information.
Pretreatment Evaluations: Evaluate for tuberculosis (TB) infection, obtain liver enzymes and bilirubin levels, and complete all age-appropriate vaccinations according to current immunization guidelines.1
Monitor: For signs and symptoms of active TB during and after treatment with TREMFYA®; liver enzymes and bilirubin levels for at least 16 weeks of treatment, and periodically thereafter according to routine patient management.1
TREMFYA® is intended for use under the guidance and supervision of a healthcare professional. TREMFYA® may be administered by a healthcare professional, or a patient/caregiver after proper training on correct subcutaneous injection technique.1
*200 mg/20 mL (10 mg/mL) solution in a single-dose vial.
†200 mg/2 mL or 100 mg/mL in a single-dose prefilled pen.
‡200 mg/2 mL or 100 mg/mL in a single-dose prefilled syringe.
§100 mg/mL in a single-dose One-Press patient-controlled injector.
IV=intravenous; q4w=every 4 weeks; q8w=every 8 weeks; SC=subcutaneous; SUBQ=subcutaneous.
*200 mg/20 mL (10 mg/mL) solution in a single-dose vial.
†200 mg/2 mL or 100 mg/mL in a single-dose prefilled pen.
‡200 mg/2 mL or 100 mg/mL in a single-dose prefilled syringe.
§100 mg/mL in a single-dose One-Press patient-controlled injector.
IV=intravenous; q4w=every 4 weeks; q8w=every 8 weeks; SC=subcutaneous; SUBQ=subcutaneous.
These videos are not meant to replace the Instructions for Use (IFU) that are supplied with TREMFYA®. Instruct patients to read the IFU before using their TREMFYA® PEN, TREMFYA® prefilled syringe, or One-Press patient-controlled injector and each time they get a refill. Please see the full IFU for TREMFYA®.






TREMFYA® is intended for use under the guidance and supervision of a healthcare professional. TREMFYA® may be administered by a healthcare professional, or a patient/caregiver after proper training on correct subcutaneous injection technique.
Reference: 1. TREMFYA® [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc.