For US Healthcare Professionals
I am a:
For US Healthcare Professionals
Full Prescribing InformationDurable response rates over time1*†
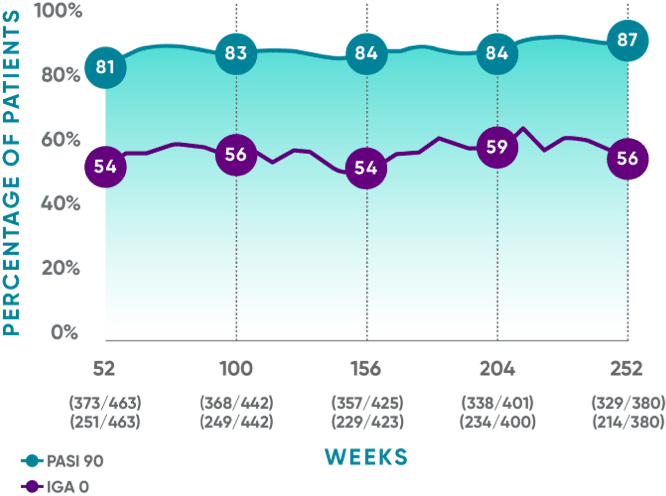
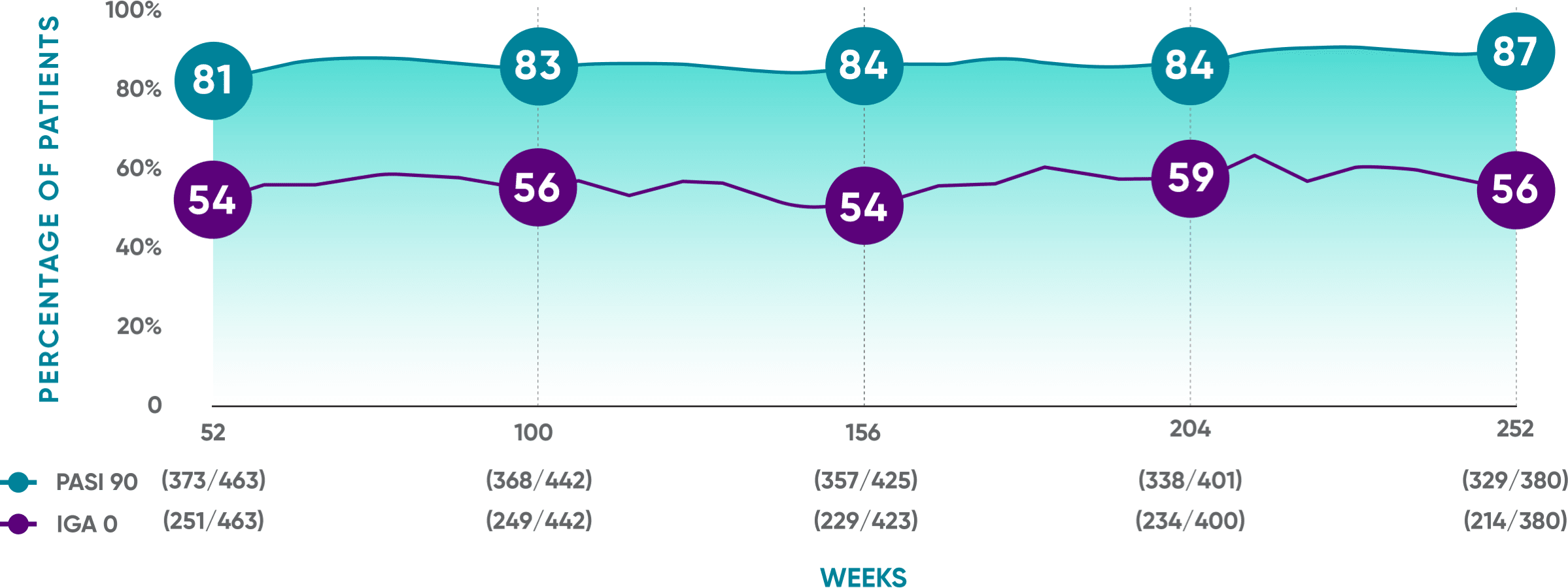
PASI 90 response rates were maintained from Week 52 through Week 252
VOYAGE 1: Prespecified secondary analysis—results through Year 5 (Week 252, as-observed analysis)*†‡


Patients who lose response or are unable to tolerate treatment are likely to discontinue treatment, which may increase the response rate in an as-observed analysis.
*The same patients may not have responded at each time point.
VOYAGE co-primary endpoints at Week 16 (NRI)1,2:
VOYAGE 1—PASI 90: TREMFYA® 73% (241/329), placebo 3% (5/174) (P<0.001). IGA 0/1: TREMFYA® 85% (280/329), placebo 7% (12/174) (P<0.001). VOYAGE 2—PASI 90: TREMFYA® 70% (347/496), placebo 2% (6/248) (P<0.001). IGA 0/1: TREMFYA® 84% (417/496), placebo 8% (21/248) (P<0.001).
Psoriasis Symptoms and Signs Diary (Week 16): Greater improvements in symptoms of psoriasis (itch, pain, stinging, burning, and skin tightness).2
In a prespecified secondary analysis of efficacy after Week 48 applying treatment failure rules (TFR)1*†‡:
- PASI 90: 80% (373/468) at Week 52; 82% (368/448) at Week 100; 83% (358/432) at Week 156; 82% (338/411) at Week 204; 84% (329/391) at Week 252
- IGA 0: 54% (251/468) at Week 52; 56% (249/448) at Week 100; 53% (229/430) at Week 156; 57% (234/410) at Week 204; 55% (214/391) at Week 252
VOYAGE 1: PASI 90 and IGA 0 at Week 48 (major secondary endpoints, NRI)2§
- 73% (84/115) of patients receiving TREMFYA® achieved PASI 90, and 47% (54/115) of patients achieved IGA 0
†Available data at each visit were used; missing data were not included in the analysis.
‡Data shown include patients randomized at Week 0 to the TREMFYA® arm and placebo-arm patients who crossed over to receive TREMFYA® at Weeks 16 and 20 and q8w thereafter.
§Results from North American sites only, which used a US-licensed active comparator. Active-comparator data not shown.
References: 1. Data on file. Janssen Biotech, Inc. 2. TREMFYA® (guselkumab) [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc.
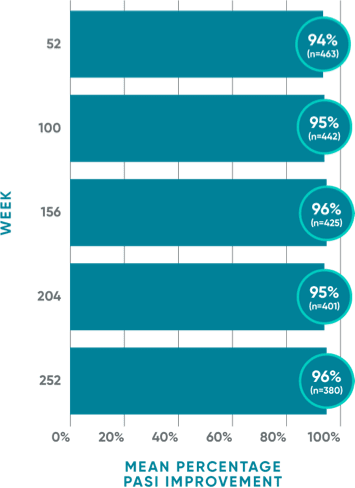
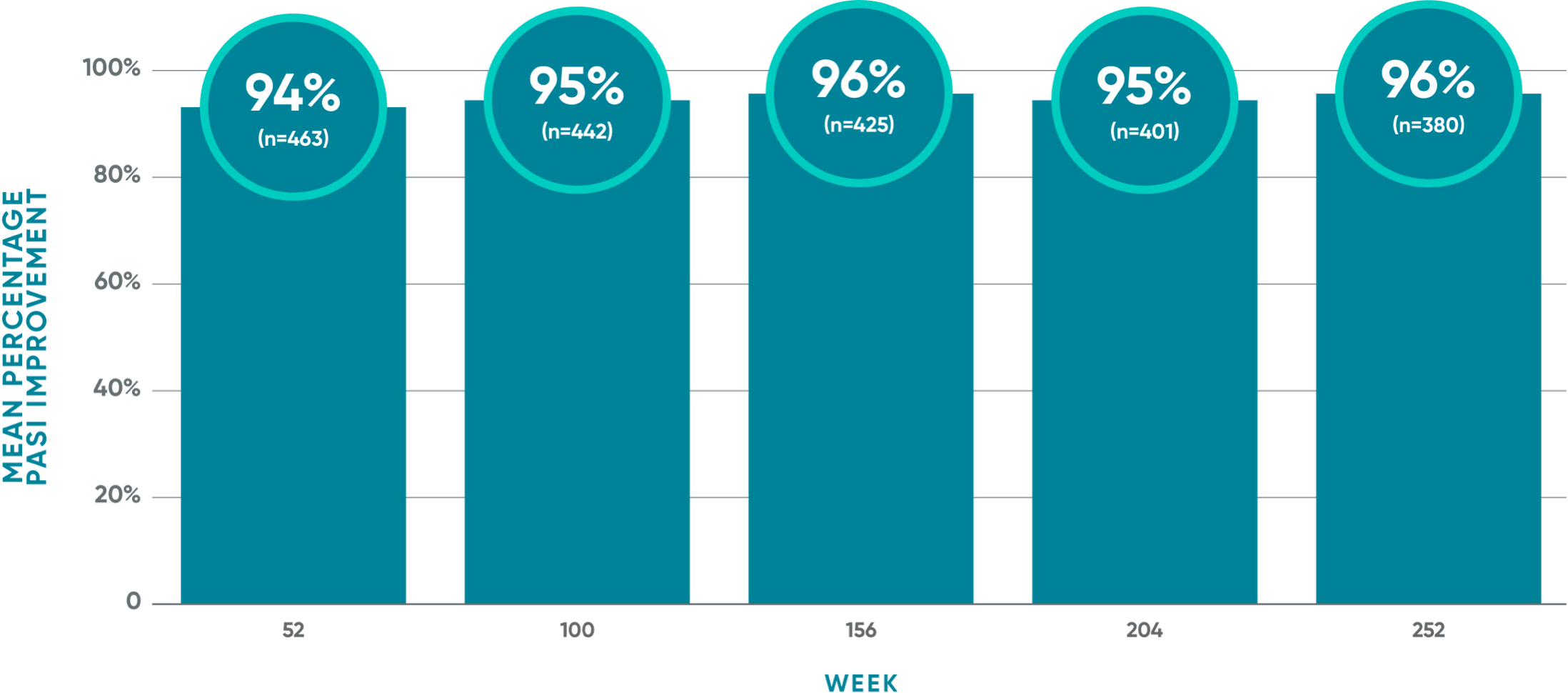
96% mean PASI improvement from baseline at Year 5 (Week 252)
VOYAGE 1: Post hoc analysis of mean PASI improvement at Week 252 (as-observed)1*†‡§


Patients who lose response or are unable to tolerate treatment are likely to discontinue treatment, which may increase the response rate in an as-observed analysis.
Patients who lose response or are unable to tolerate treatment are likely to discontinue treatment, which may increase the mean PASI percentage improvement in an as-observed analysis.
*The same patients may not have responded at each time point.
VOYAGE co-primary endpoints at Week 16 (NRI)1,2:
VOYAGE 1—PASI 90: TREMFYA® 73% (241/329), placebo 3% (5/174) (P<0.001). IGA 0/1: TREMFYA® 85% (280/329), placebo 7% (12/174) (P<0.001). VOYAGE 2—PASI 90: TREMFYA® 70% (347/496), placebo 2% (6/248) (P<0.001). IGA 0/1: TREMFYA® 84% (417/496), placebo 8% (21/248) (P<0.001).
Psoriasis Symptoms and Signs Diary (Week 16): Greater improvements in symptoms of psoriasis (itch, pain, stinging, burning, and skin tightness).2
VOYAGE 1: Prespecified other secondary analysis–mean PASI improvement from baseline (TFR)1*†‡§
- Mean PASI improvement from baseline with TREMFYA® was 93% at Week 52 (n=468), 94% at Week 100 (n=448), 94% at Week 156 (n=432), 93% at Week 204 (n=411), and 93% at Week 252 (n=391)
Mean PASI improvement is an assessment of the average percentage improvement from baseline in psoriatic signs of redness, thickness, scale, and body surface area of involvement.
†Data shown include patients randomized at Week 0 to TREMFYA® arm and placebo arm patients who crossed over to receive TREMFYA® at Weeks 16 and 20 and q8w thereafter.
‡Available data at each visit were used; missing data were not included in the analysis.
§TFR methods: Patients who discontinued study agent due to lack of efficacy or an adverse event of worsening of psoriasis, or who started a protocol-prohibited medication, including conventional and biologic systemic therapy, phototherapy, and/or ultra–high-potency corticosteroids were considered treatment failures. Other topical agents for psoriasis were permitted. Patients were considered to have no improvement (percentage improvement=0) after meeting treatment failure criteria.
References: 1. Data on file. Janssen Biotech, Inc. 2. TREMFYA® (guselkumab) [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc.


