For US Healthcare Professionals
I am a:
For US Healthcare Professionals
Full Prescribing InformationCompletely
Clear
PsO
At Week 24, 53% of patients in VOYAGE 1 and 48% of patients in VOYAGE 2 achieved IGA 0.1,2
Consistent &
Durable
PsO
In VOYAGE 1, PASI 90 response rates were consistent between doses from Weeks 20−48. In the open-label extension, response rates were maintained from Weeks 52−252.2,3*
Sustained
Joint
Improvement†
PsA
A majority of patients achieved ACR20 response at Week 24 in both DISCOVER 1 (52%) and DISCOVER 2 (64%). In the DISCOVER 2 open-label extension, ACR20 response rates were sustained from Weeks 52-100.1,3-5*‡
Choose
TREMFYA®.
Get
TREMFYA®.
PsO
Nearly 100% of commercially insured patients have preferred§ access to TREMFYA®.||¶
*Patients who lose response or are unable to tolerate treatment are likely to discontinue treatment, which may increase the response rate in an as-observed analysis. The same patients may not have responded at each time point.
†Based on ACR response rates.
‡After Week 24, patients and doctors knew that all patients were on TREMFYA® (open label with a blinded dosing interval), which may have affected the results.
§Preferred means TREMFYA® is available on the plan’s formulary and may require a step edit.
||Within 2% of actual access.
¶This may not represent 100% of lives due to data limitations.
IGA=Investigator’s Global Assessment, IGA score of cleared (0) or minimal (1) using a 5-point scale of overall disease severity; PASI=Psoriasis Area and Severity Index.
Once a prescribing decision has been made



Once a prescribing decision has been made

A support program that offers a range of dedicated support and resources to help make it easier for patients as they begin, and continue, their TREMFYA® treatment journey.
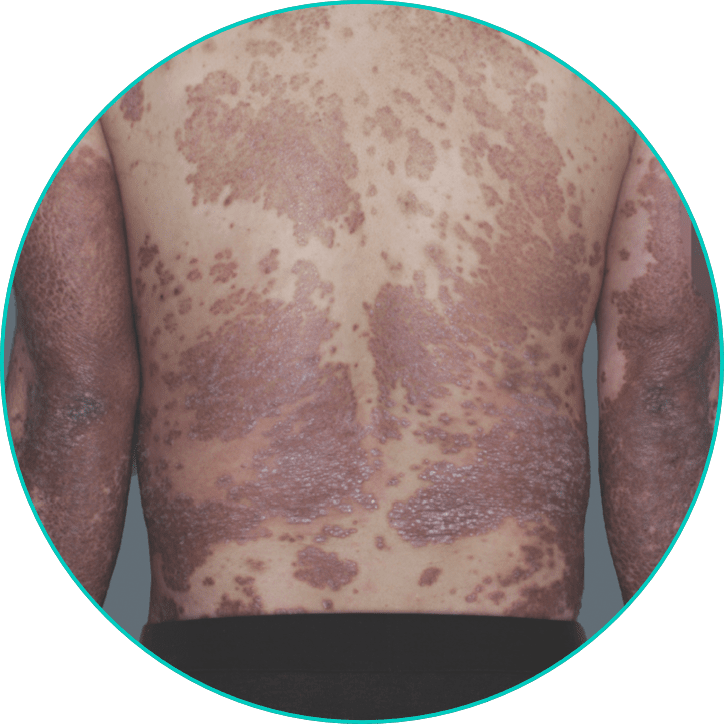
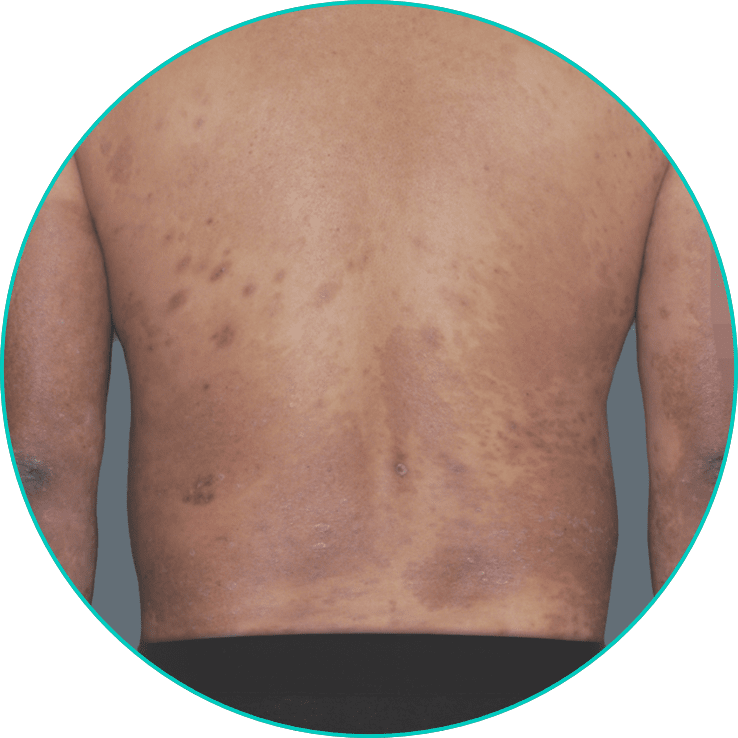
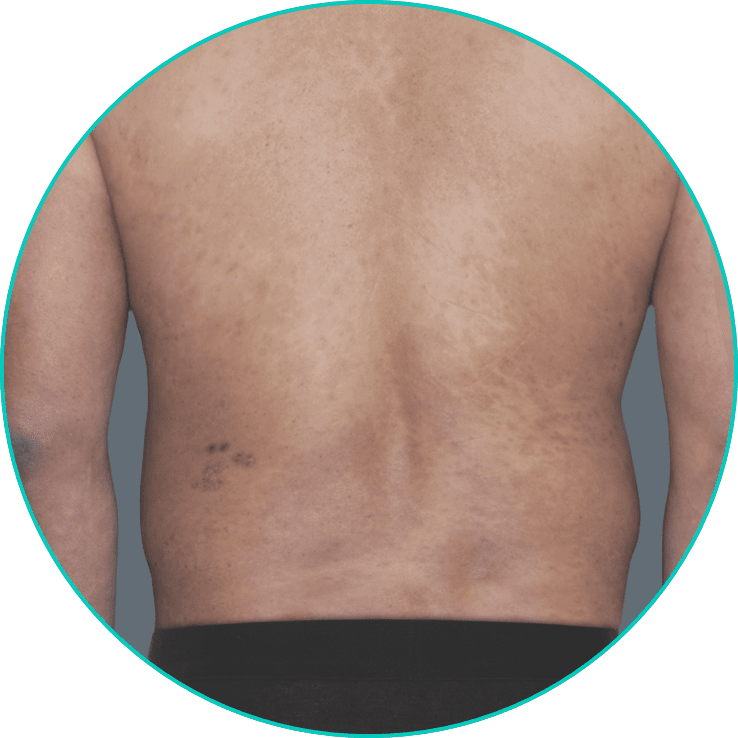
See a patient on TREMFYA® get clearer skin3*
*Individual results may vary. Plaque psoriasis photos are of real patients from VOYAGE 2 who received TREMFYA® 100 mg at Weeks 0 and 4, and q8w thereafter through Week 48. Other areas affected (not shown) yielded similar results to those depicted here. Images are for illustrative purposes only and are used with permission from Janssen Biotech, Inc.
The only IL-23 inhibitor with the One-Press patient-controlled injector
Optimized dosing
The TREMFYA® 8-week dosing schedule was optimized in clinical trials1,6

Controlled delivery
Patients inject at a speed that is comfortable for them
Not actual size.
TREMFYA® is administered as a 100-mg subcutaneous injection once every 8 weeks, after starter doses at Weeks 0 and 4. TREMFYA® is intended for use under the guidance and supervision of a physician. Patients may self-inject with TREMFYA® after physician approval and proper training. In active PsA, TREMFYA® may be administered alone or in combination with a conventional DMARD (eg, methotrexate).
DMARD=disease-modifying antirheumatic drug.
References: 1. TREMFYA® (guselkumab) [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc. 2. Blauvelt A, Papp KA, Griffiths CEM, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76(3):405-417. 3. Data on file. Janssen Biotech, Inc. 4. Mease PJ, Rahman P, Gottlieb AB, et al; DISCOVER-2 Study Group. Guselkumab in biologic-naive patients with active psoriatic arthritis (DISCOVER-2): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395(10230):1126-1136. 5. Deodhar A, Helliwell PS, Boehncke W-H, et al. Guselkumab in patients with active psoriatic arthritis who were biologic-naïve or had previously received TNFα inhibitor treatment (DISCOVER 1): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395(10230):1115-1125. 6. Lebwohl M, Langley RG, Zhu Y, et al. Use of dose-exposure-response relationships in Phase 2 and Phase 3 guselkumab studies to optimize dose selection in psoriasis. J Eur Acad Dermatol Venereol. 2019;33(11):2082-2086.