For US Healthcare Professionals
I am a:
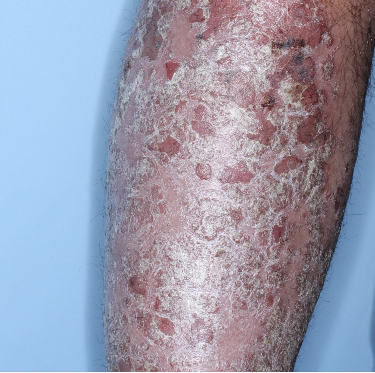
Patients saw clearer skin at Week 16 and Week 481,2
Week 0
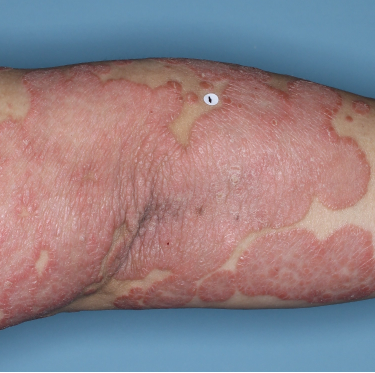
PASI=20.6
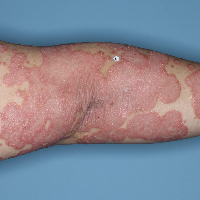
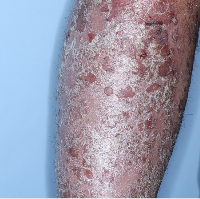
Week 16
PASI=2.9
(86% PASI
improvement)
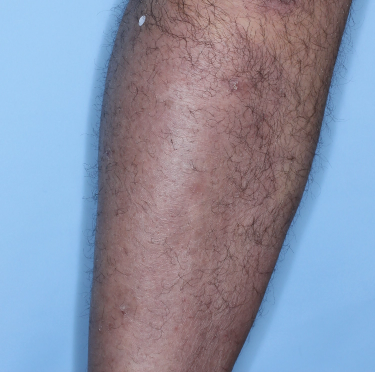
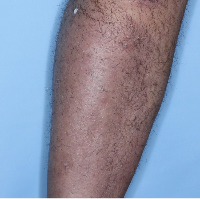
Week 48
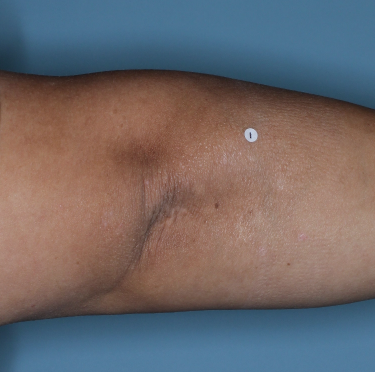
PASI=1.10
(95% PASI
improvement)
Individual results may vary.
Images are Janssen-owned from blinded trial: NCT05272150.
VOYAGE 1: Major secondary endpoints PASI 90 response at Weeks 16, 24, and 48 (NRI)*
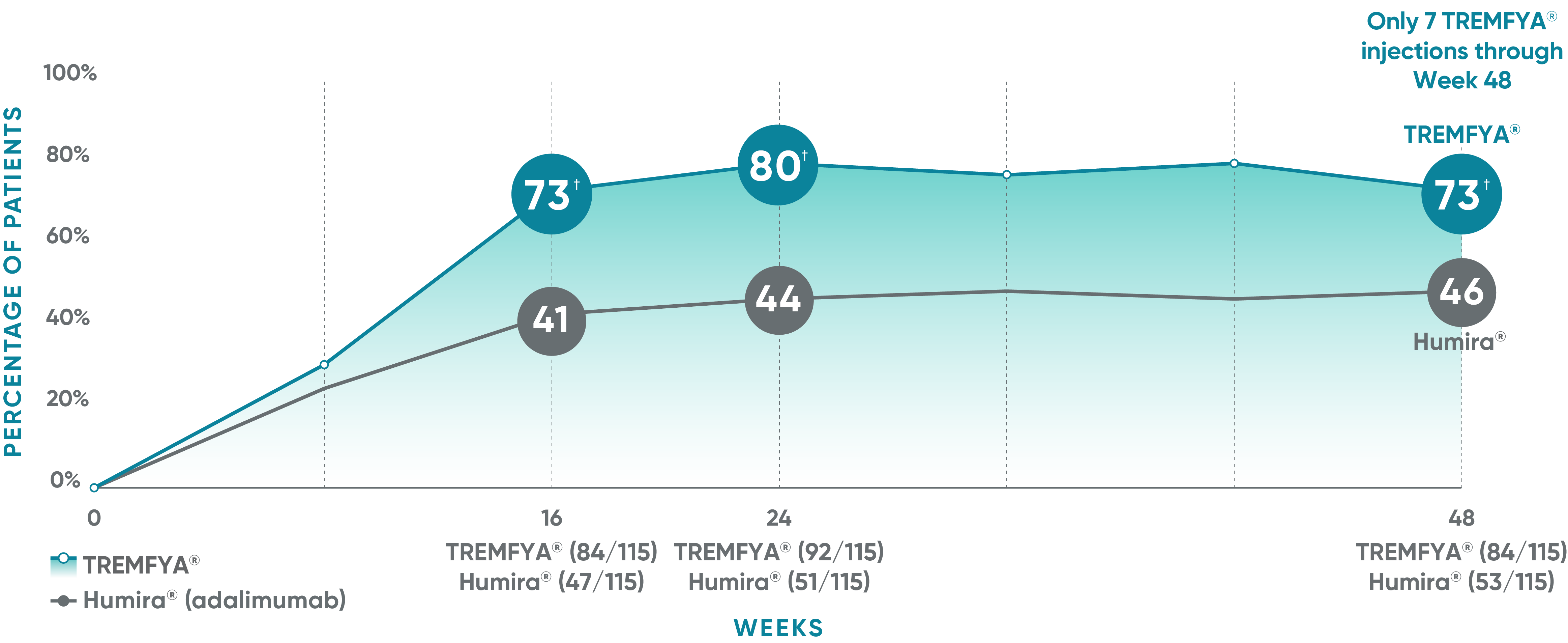


The same patients may not have responded at each time point.
VOYAGE co-primary endpoints at Week 16 (NRI)1,2:
VOYAGE 1—PASI 90: TREMFYA® 73% (241/329), placebo 3% (5/174) (P<0.001). IGA 0/1: TREMFYA® 85% (280/329), placebo 7% (12/174) (P<0.001). VOYAGE 2—PASI 90: TREMFYA® 70% (347/496), placebo 2% (6/248) (P<0.001). IGA 0/1: TREMFYA® 84% (417/496), placebo 8% (21/248) (P<0.001).
VOYAGE 2: Major secondary endpoint at Week 16 (NRI)1,2*†
- 64% (102/160) of patients receiving TREMFYA® achieved PASI 90 vs 42% (34/81) of patients receiving Humira®
VOYAGE 2: Major secondary endpoint at Week 24 (NRI)1,2*‡
- 71% (113/160) of patients receiving TREMFYA® achieved PASI 90 vs 51% (41/81) of patients receiving Humira®
*Results from North American sites only, which used US-licensed Humira®.
†P<0.001 vs Humira®.
‡P=0.003 vs Humira®.
Humira® is a registered trademark of Abbvie Biotechnology Ltd. Corporation.
NRI=nonresponder imputation.
References: 1. Data on file. Janssen Biotech, Inc. 2. TREMFYA® (guselkumab) [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc.
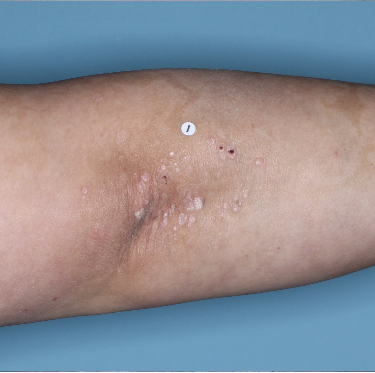
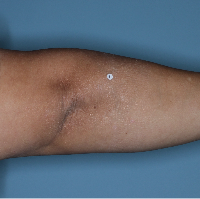
48% of patients were completely clear* at Week 481
Week 0
PASI=20.1
Week 16
PASI=0
(100% PASI
improvement)
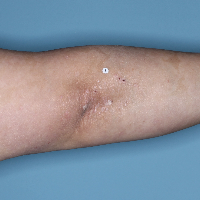
Week 48
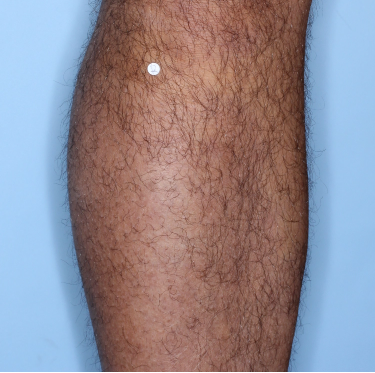
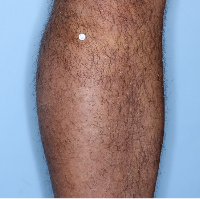
PASI=0
(100% PASI
improvement)
Individual results may vary.
Images are Janssen-owned from blinded trial: NCT05272150.
VOYAGE 1: Post hoc analysis PASI 100 response at Weeks 16, 24, and 48 (NRI)*†
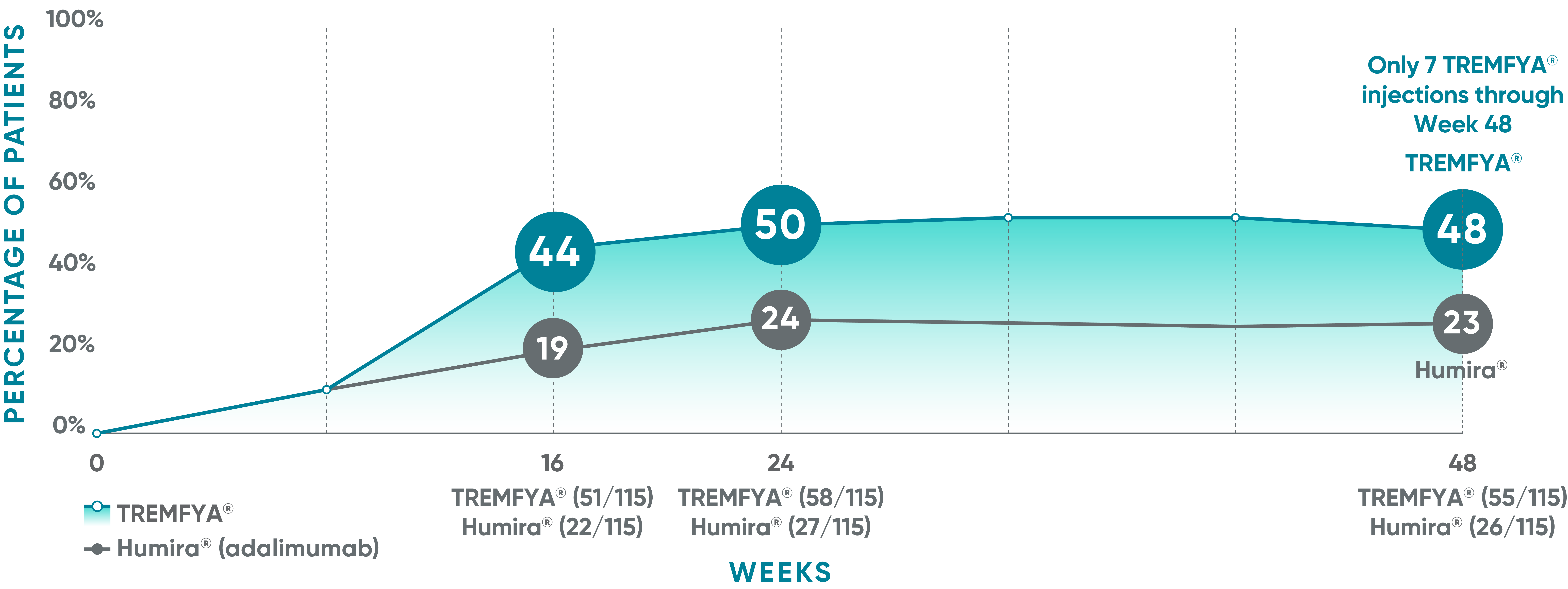


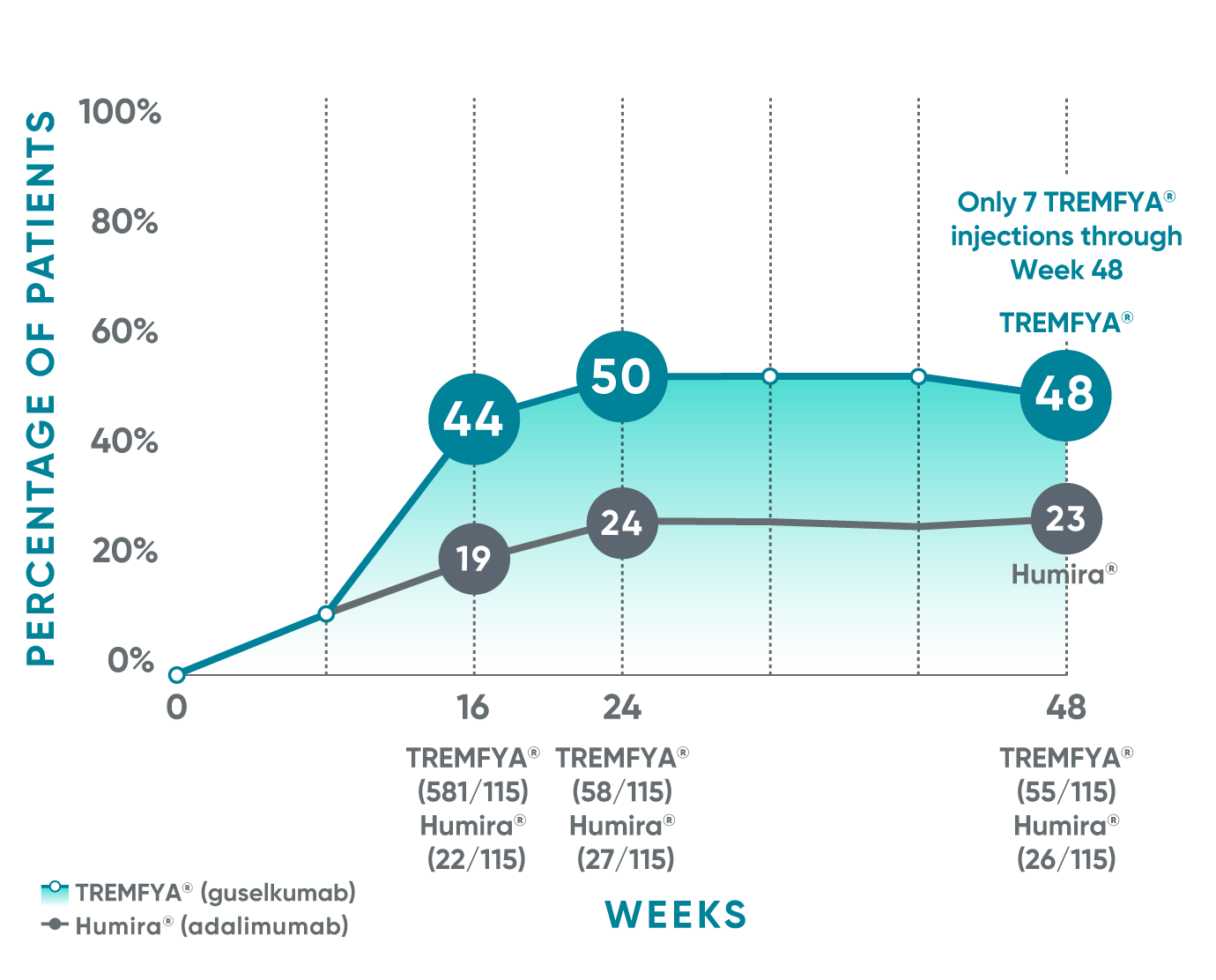
*PASI 100 was a post hoc analysis that was not adjusted for multiplicity: P values were considered nominal.
The same patients may not have responded at each time point.
VOYAGE co-primary endpoints at Week 16 (NRI)1,2:
VOYAGE 1—PASI 90: TREMFYA® 73% (241/329), placebo 3% (5/174) (P<0.001). IGA 0/1: TREMFYA® 85% (280/329), placebo 7% (12/174) (P<0.001).
VOYAGE 2—PASI 90: TREMFYA® 70% (347/496), placebo 2% (6/248) (P<0.001). IGA 0/1: TREMFYA® 84% (417/496), placebo 8% (21/248) (P<0.001).
†Results from North American sites only, which used US-licensed Humira®.
Humira is a registered trademark of Abbvie Biotechnology Ltd. Corporation.
NRI=nonresponder imputation; PASI 100=proportion of patients who achieved 100% reduction (or improvement) in PASI score from baseline.
References: 1. Data on file. Janssen Biotech, Inc. 2. TREMFYA® (guselkumab) [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc.
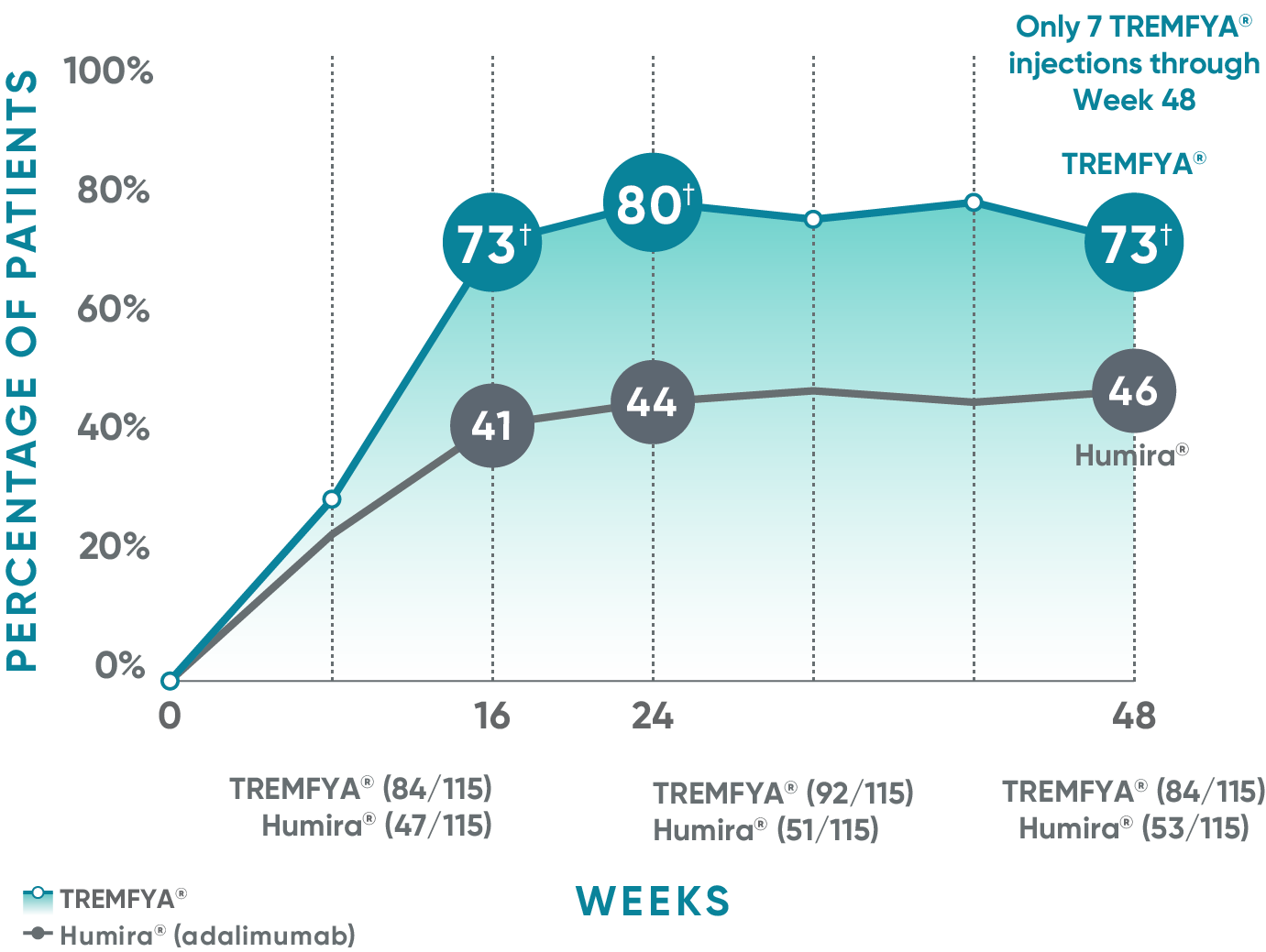
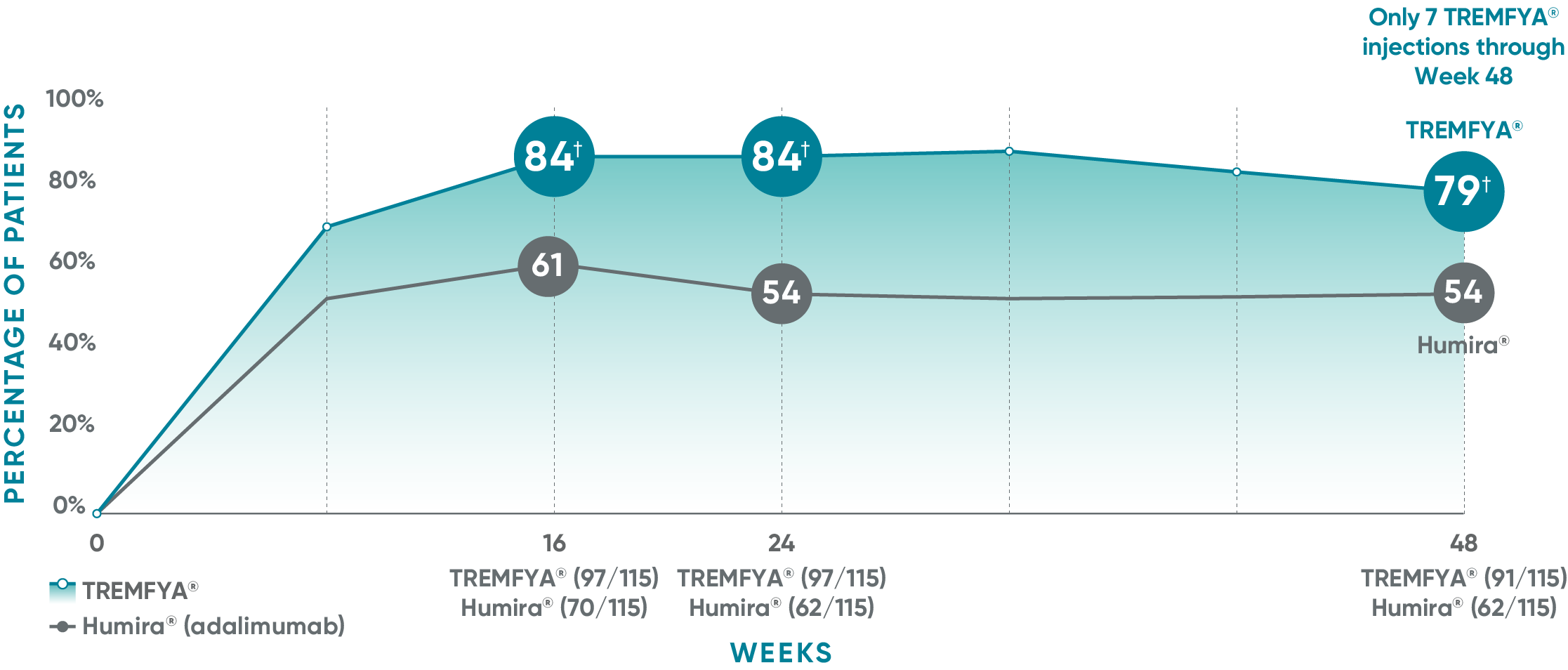
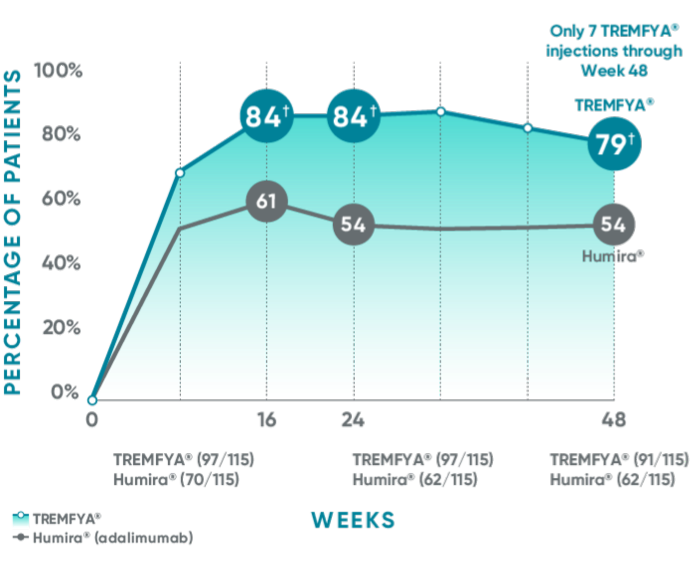
Consistent efficacy, dose to dose
VOYAGE 1: Prespecified secondary analysis—PASI 90 response rates through Week 48 (NRI)1,2
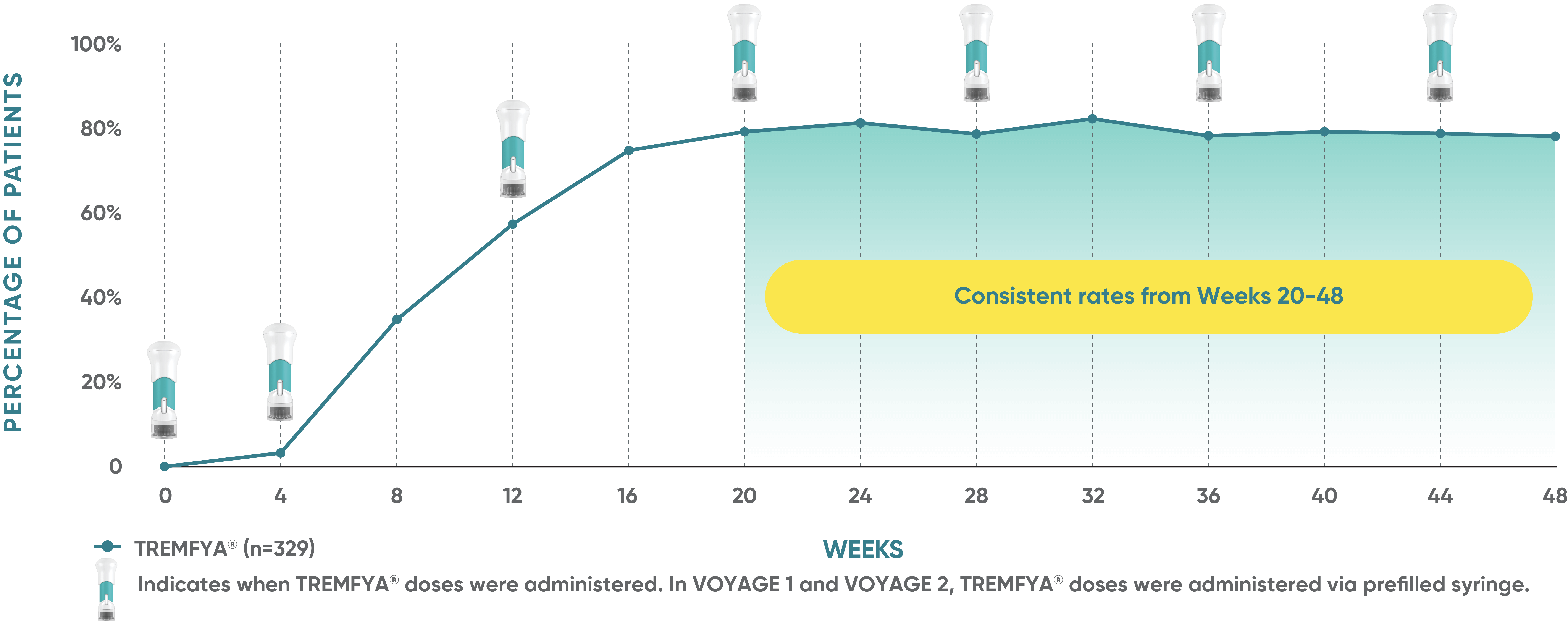
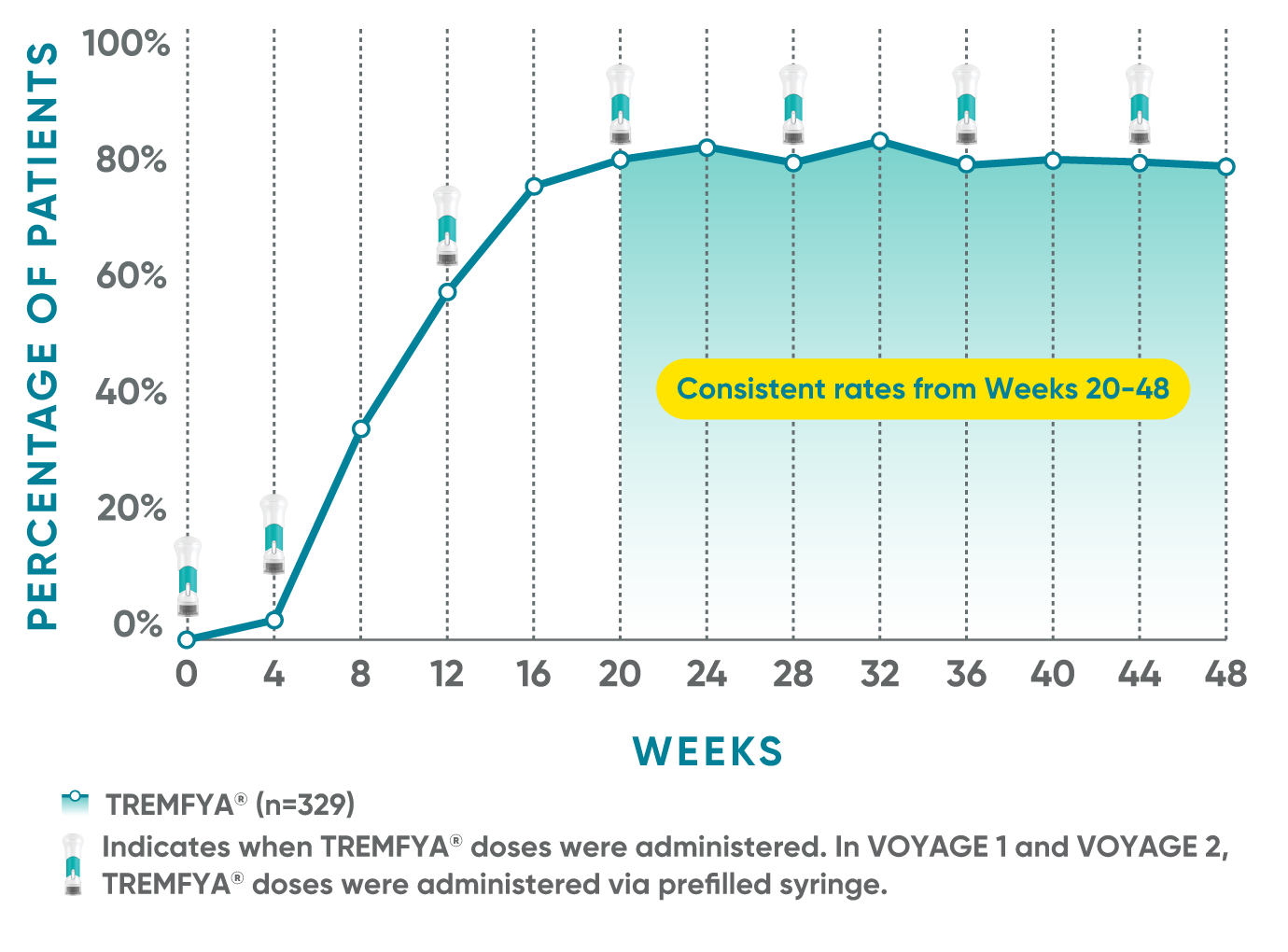
Data shown include patients randomized at Week 0 to the TREMFYA® arm.
The same patients may not have responded at each time point.
Placebo and active-comparator data are not shown in chart.
VOYAGE co-primary endpoints at Week 16 (NRI)2,3:
VOYAGE 1—PASI 90: TREMFYA® 73% (241/329), placebo 3% (5/174) (P<0.001). IGA 0/1: TREMFYA® 85% (280/329), placebo 7% (12/174) (P<0.001).
VOYAGE 2—PASI 90: TREMFYA® 70% (347/496), placebo 2% (6/248) (P<0.001). IGA 0/1: TREMFYA® 84% (417/496), placebo 8% (21/248) (P<0.001).
VOYAGE 2: PASI 90 at Weeks 16 and 24 (major secondary endpoints, NRI)3*
- 64% (102/160) of patients receiving TREMFYA® achieved PASI 90 response at Week 16
- 71% (113/160) of patients receiving TREMFYA® achieved PASI 90 response at Week 24
*Results from North American sites only, which used US-licensed Humira®.
Dosing and administration
- 100 mg administered by subcutaneous injection at Week 0, Week 4, and every 8 weeks thereafter3
References: 1. Blauvelt A, Papp KA, Griffiths CEM, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76(3):405-417. 2. Data on file. Janssen Biotech, Inc. 3. TREMFYA® (guselkumab) [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc.
The majority of patients saw clearer skin with TREMFYA®1,2
Week 0
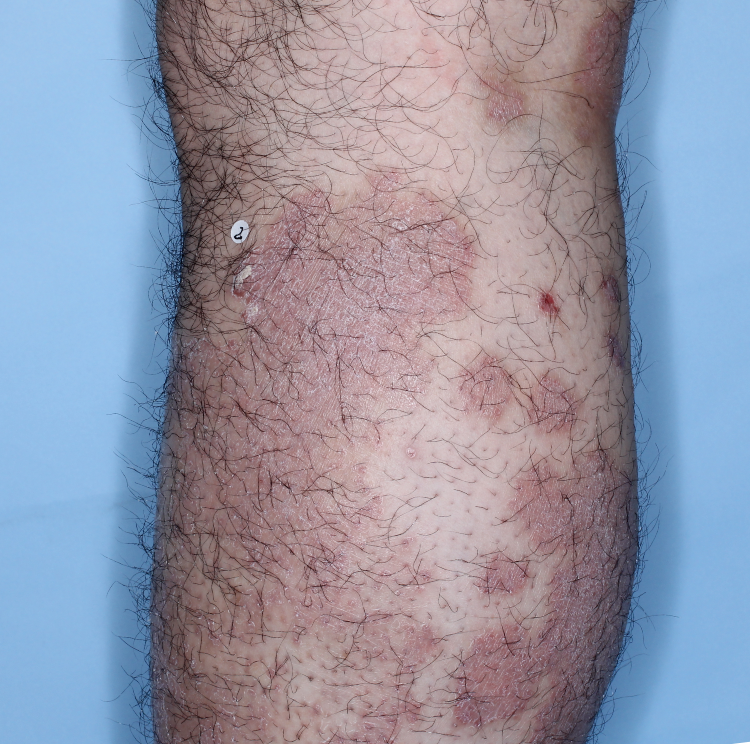

IGA=3
Week 16
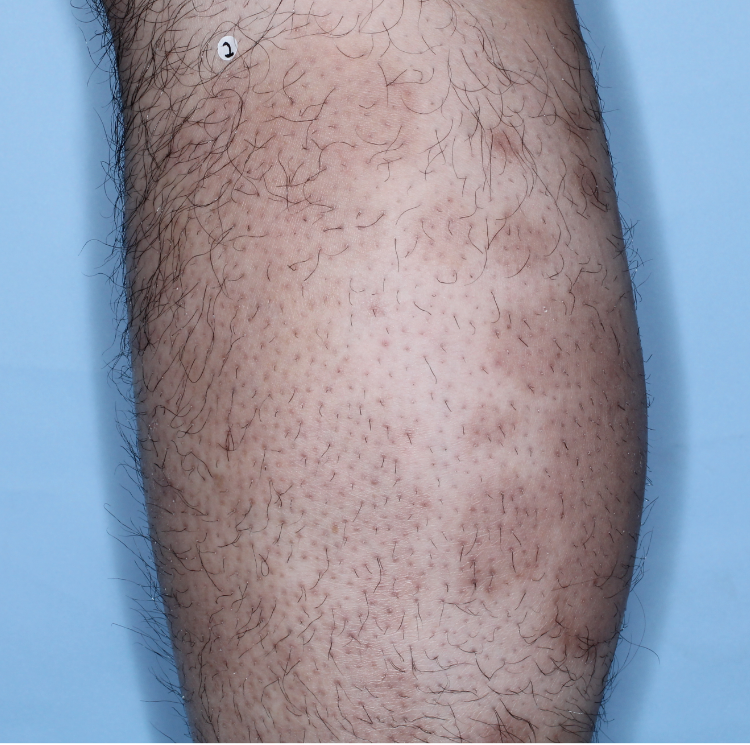

IGA=2
Week 48
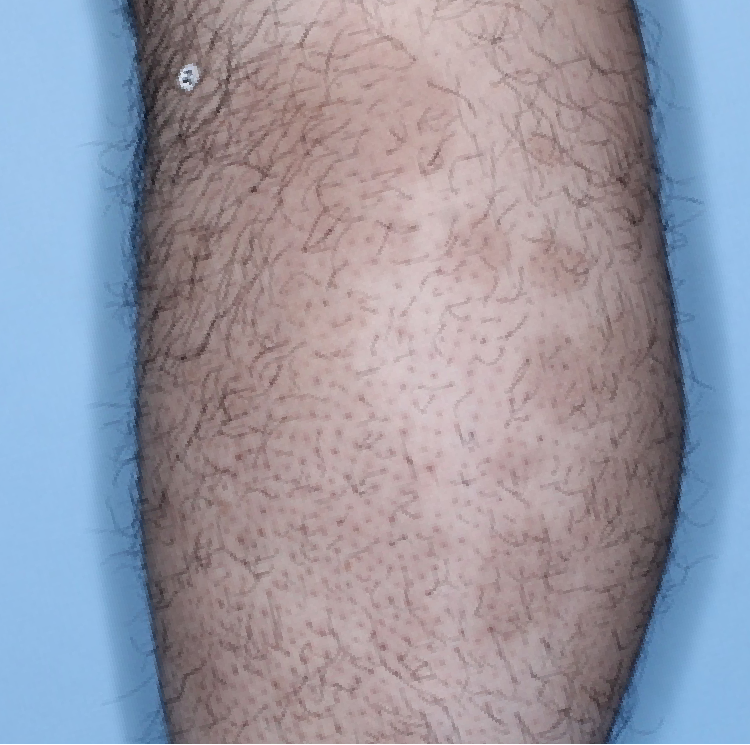

IGA=1
Individual results may vary.
Images are Janssen-owned from blinded trial: NCT05272150.
VOYAGE 1: Major secondary endpoints IGA 0/1 at Weeks 16, 24, and 48 (NRI)*


The same patients may not have responded at each time point.
VOYAGE co-primary endpoints at Week 16 (NRI)1,2:
VOYAGE 1—PASI 90: TREMFYA® 73% (241/329), placebo 3% (5/174) (P<0.001). IGA 0/1: TREMFYA® 85% (280/329), placebo 7% (12/174) (P<0.001).
VOYAGE 2—PASI 90: TREMFYA® 70% (347/496), placebo 2% (6/248) (P<0.001). IGA 0/1: TREMFYA® 84% (417/496), placebo 8% (21/248) (P<0.001).
VOYAGE 2: Major secondary endpoint at Week 16 (NRI)1,2*‡
- 74% (119/160) of patients receiving TREMFYA® achieved IGA 0/1 vs 62% (50/81) of patients receiving Humira®
VOYAGE 2: Major secondary endpoint at Week 24 (NRI)1,2*§
- 74% (119/160) of patients receiving TREMFYA® achieved IGA 0/1 vs 57% (46/81) of patients receiving Humira®
Humira is a registered trademark of Abbvie Biotechnology Ltd. Corporation.
Nonresponder imputation (NRI) methods were used for analysis.
*Results from North American sites only, which used US-licensed Humira®.
†P=0.001 vs Humira®.
‡P=0.027 vs Humira®.
§P=0.005 vs Humira®.
References: 1. Data on file. Janssen Biotech, Inc. 2. TREMFYA® (guselkumab) [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc.
TREMFYA® complete clearance results at Week 16 and Week 481,2
Week 0
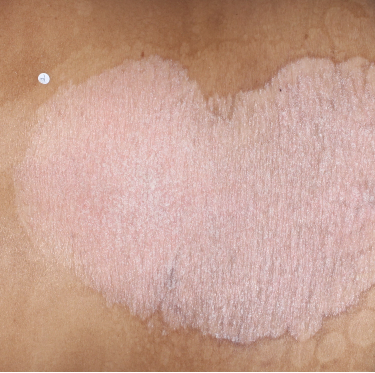
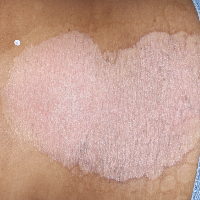
IGA=4
Week 16
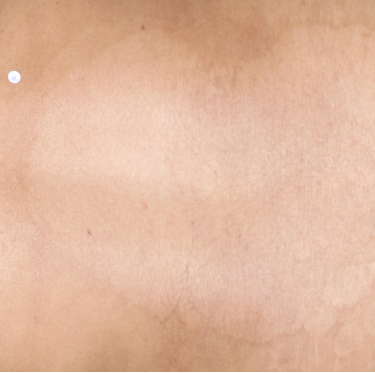
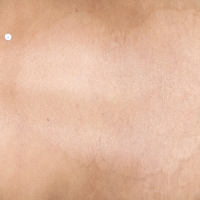
IGA=0
Week 48
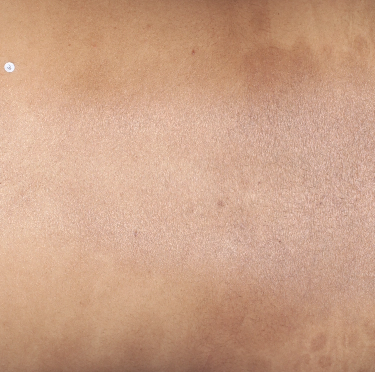
IGA=0
Individual results may vary.
Images are Janssen-owned from blinded trial: NCT05272150.
VOYAGE 1: Major secondary endpoints IGA 0 at Weeks 24 and 48* (NRI)
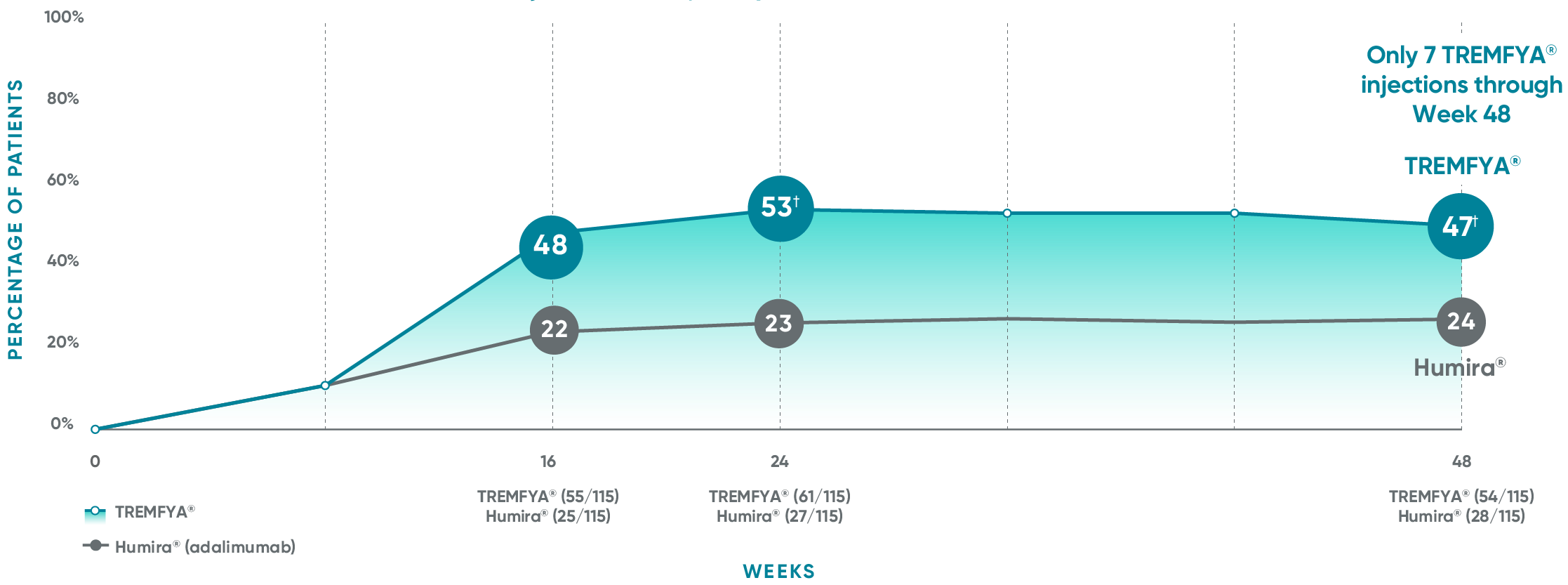


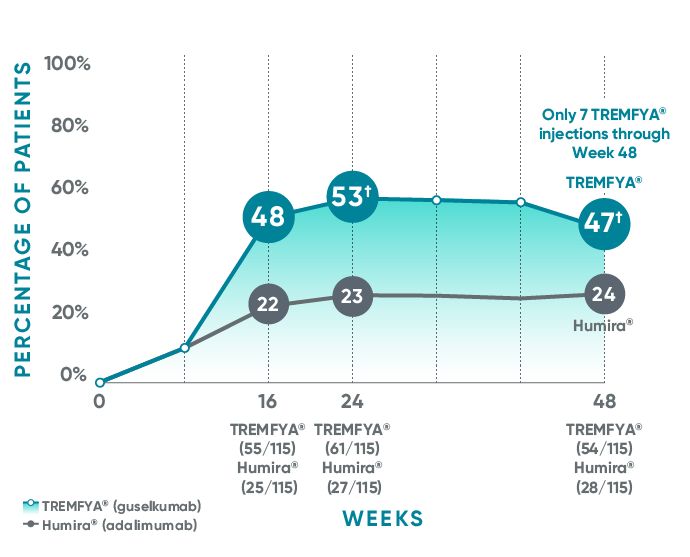
The same patients may not have responded at each time point.
IGA 0 at Week 16 was from a post hoc analysis that was not adjusted for multiplicity. P value was considered nominal.
VOYAGE co-primary endpoints at Week 16 (NRI)1,2:
VOYAGE 1—PASI 90: TREMFYA® 73% (241/329), placebo 3% (5/174) (P<0.001). IGA 0/1: TREMFYA® 85% (280/329), placebo 7% (12/174) (P<0.001).
VOYAGE 2—PASI 90: TREMFYA® 70% (347/496), placebo 2% (6/248) (P<0.001). IGA 0/1: TREMFYA® 84% (417/496), placebo 8% (21/248) (P<0.001).
VOYAGE 2: Major secondary endpoint at Week 24 (NRI)1,2*†
- 48% (76/160) of patients receiving TREMFYA® achieved IGA 0 compared with 28% (23/81) of patients receiving Humira®
(P=0.005)
†Results from North American sites only, which used a US-licensed active comparator.
‡P<0.001 vs Humira®.
NRI=nonresponder imputation.
References: 1. Data on file. Janssen Biotech, Inc. 2. TREMFYA® (guselkumab) [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc.
IN ADULTS WITH MODERATE TO SEVERE PLAQUE PsO


