For US Healthcare Professionals
I am a:
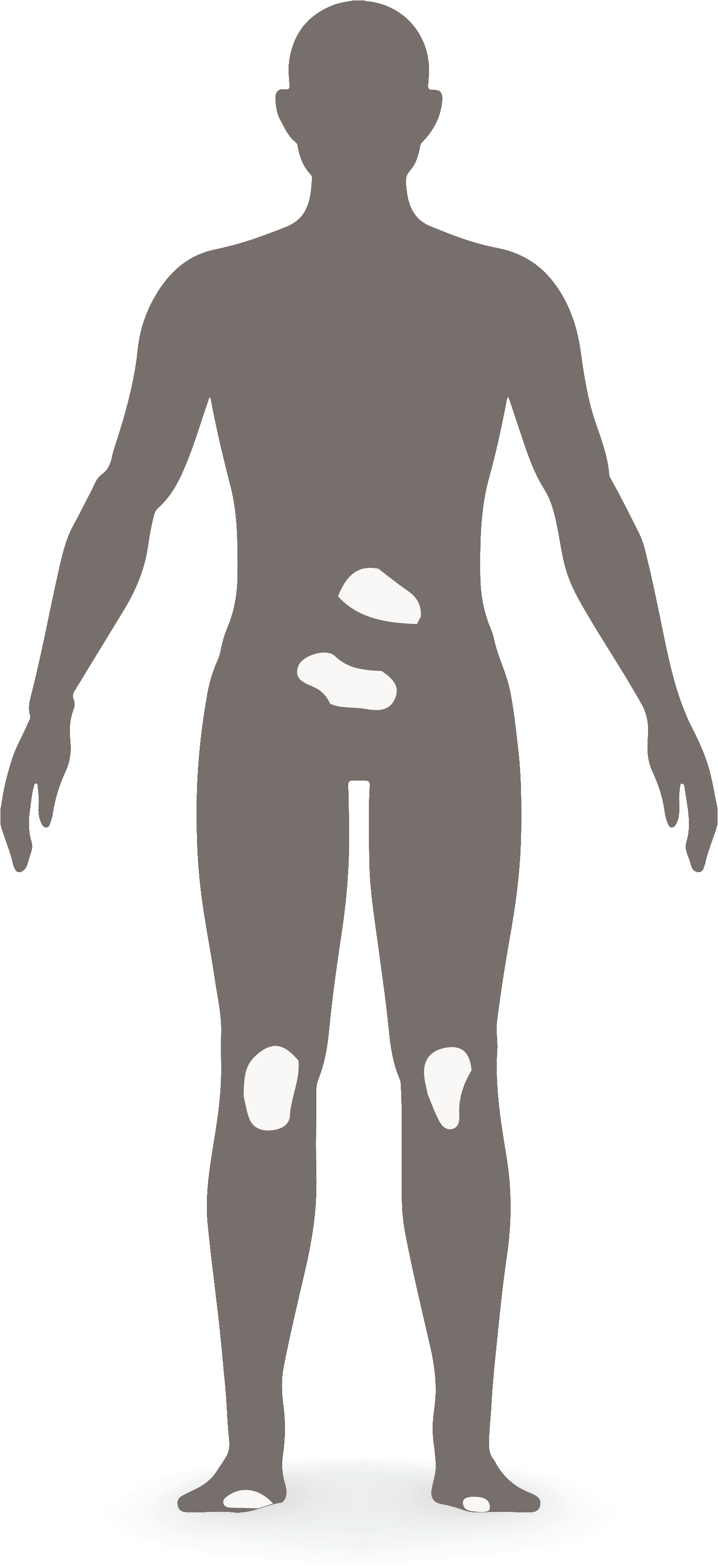
A small area doesn’t always mean a small burden
Psoriasis (PsO) is traditionally evaluated by body surface area (BSA), but low-BSA involvement can still have a significant impact on symptoms. Patients with low-BSA and high-impact site involvement often remain untreated—BSA alone should no longer be the only trigger for systemic therapy, including biologics.
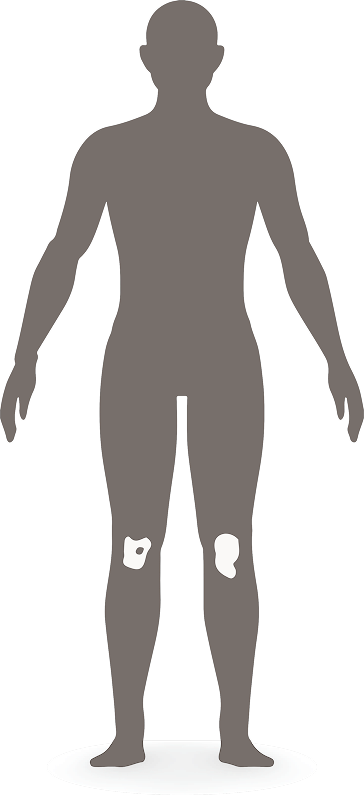
Mild
<3% BSA1
Moderate
3-10% BSA1

Severe
>10% BSA1

of patients with BSA of 3%-10% have 1 or more high-impact site involvement2†

of systemic therapy-naïve PsO patients with ≤10% BSA and high-impact site involvement did not see symptom resolution with topicals3‡
IPC guidelines advocate that patients who have high-impact site involvement or who have failed topical therapies are eligible for systemic therapies, including biologics, regardless of BSA.1
*BSA criteria of 2%-15%.
†Based on one retrospective cohort study using an Electronic Health Record dataset.
‡Based on an online survey of 175 patients with PsO (BSA ≤10%). Limitations of the study include potential bias in cohort selection and outcomes reporting, generalizability of the study, and recall bias. Since statistical analysis was not conducted, no causal conclusions can be drawn.
IPC=International Psoriasis Council.
References: 1. Strober B, Ryan C, van de Kerkhof P, et al. Recategorization of psoriasis severity: Delphi consensus from the International Psoriasis Council. J Am Acad Dermatol.
2020;82(1):117-122. 2. Horner ME, Orroth KK, Ma J, et al. Redefining disease severity with special area involvement and reflecting on treatment patterns in a real-world psoriasis population. Dermatol Ther. 2024;14:187-199. 3. Gupta S, Garbarini S, Nazareth T, et al. Characterizing outcomes and unmet needs among patients in the United States with mild-to-moderate plaque psoriasis using prescription topicals. Dermatol Ther. 2021;11:2057-2075.
IN ADULT PATIENTS WITH MODERATE PLAQUE PsO
TREMFYA® demonstrated significant skin clearance in
patients with low-BSA* and high-impact site involvement1-3
Skin clearance at Week 16
Week 0
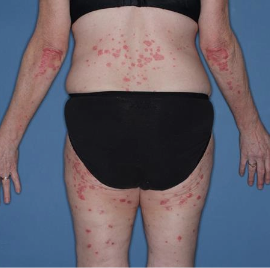
IGA=3
Week 16
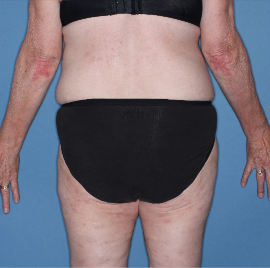
IGA=2
Actual patient from the VISIBLE study.
Individual results may vary.
Images are Janssen-owned from blinded trial: NCT05272150.
SPECTREM: IGA 0/1 primary
endpoint (NRI)
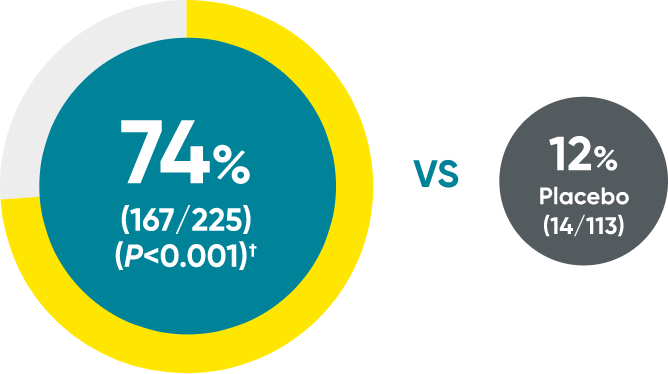
Complete clearance at Week 16
Week 0
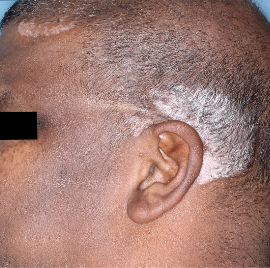
IGA=3
Week 16
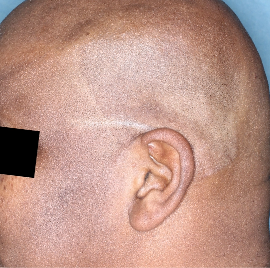
IGA=0
SPECTREM: IGA 0 major
secondary endpoint (NRI)
Actual patients from the SPECTREM study. Individual results may vary.
Images are Janssen-owned from blinded trial: NCT06039189.
SPECTREM: IGA 0/1 primary endpoint (NRI)

SPECTREM: IGA 0 major secondary endpoint (NRI)

VOYAGE 1 and VOYAGE 2 co-primary endpoints at Week 16 (NRI)4-6:
VOYAGE 1—PASI 90: TREMFYA® 73% (241/329), placebo 3% (5/174) (P<0.001). IGA 0/1: TREMFYA® 85% (280/329), placebo 7% (12/174) (P<0.001). VOYAGE 2—PASI 90: TREMFYA® 70% (347/496), placebo 2% (6/248) (P<0.001). IGA 0/1: TREMFYA® 84% (417/496), placebo 8% (21/248) (P<0.001).
*BSA criteria of 2%-15%.
†P value is based on the Cochran-Mantel-Haenszel (CMH) test stratified by special site (scalp, face, intertriginous, genital).
BSA=body surface area; IGA=Investigator’s Global Assessment; NRI=nonresponder imputation.
References: 1. Data on file. Janssen Biotech, Inc. 2. Stein Gold
L, Strober B, Armstrong AW, et al. SPECTREM: guselkumab demonstrates consistent significant clearance at
week 16 across the full range of low body surface area,
moderate psoriasis with special sites involvement. Poster
presented at: 2024 Fall Clinical Dermatology Conference;
October 24-27, 2024; Las Vegas, NV. 3. Glick BP, Beeker J,
Alonso-Llamazares J, et al. SPECTREM: guselkumab demonstrates consistent complete clearance at Week 16
across
special sites in participants with low body surface
area, moderate psoriasis. Poster presented at: 2024 Fall
Clinical Dermatology Conference; October 24-27, 2024; Las
Vegas, NV.
4. TREMFYA® (guselkumab) [Prescribing
Information]. Horsham, PA: Janssen Biotech, Inc. 5. Blauvelt A,
Papp KA, Griffiths CEM, et al. Efficacy and safety of
guselkumab, an anti-
interleukin-23 monoclonal antibody,
compared with adalimumab for the continuous treatment of
patients with moderate to severe psoriasis: results from the
phase III, double-
blinded, placebo- and active comparator–
controlled VOYAGE 1 trial. J Am Acad Dermatol.
2017;76(3):405-417. 6. Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of
guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized
withdrawal and retreatment: results from the
phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol.
2017;76(3):418-431.
IN ADULT PATIENTS WITH MODERATE PLAQUE PSORIASIS
Skin clearance rates across some of the most prevalent
high-impact sites1-4
SPECTREM: Major secondary
endpoints at Week 16 with a low-BSA*
respective high-impact site
score ≥3
at baseline (NRI)†

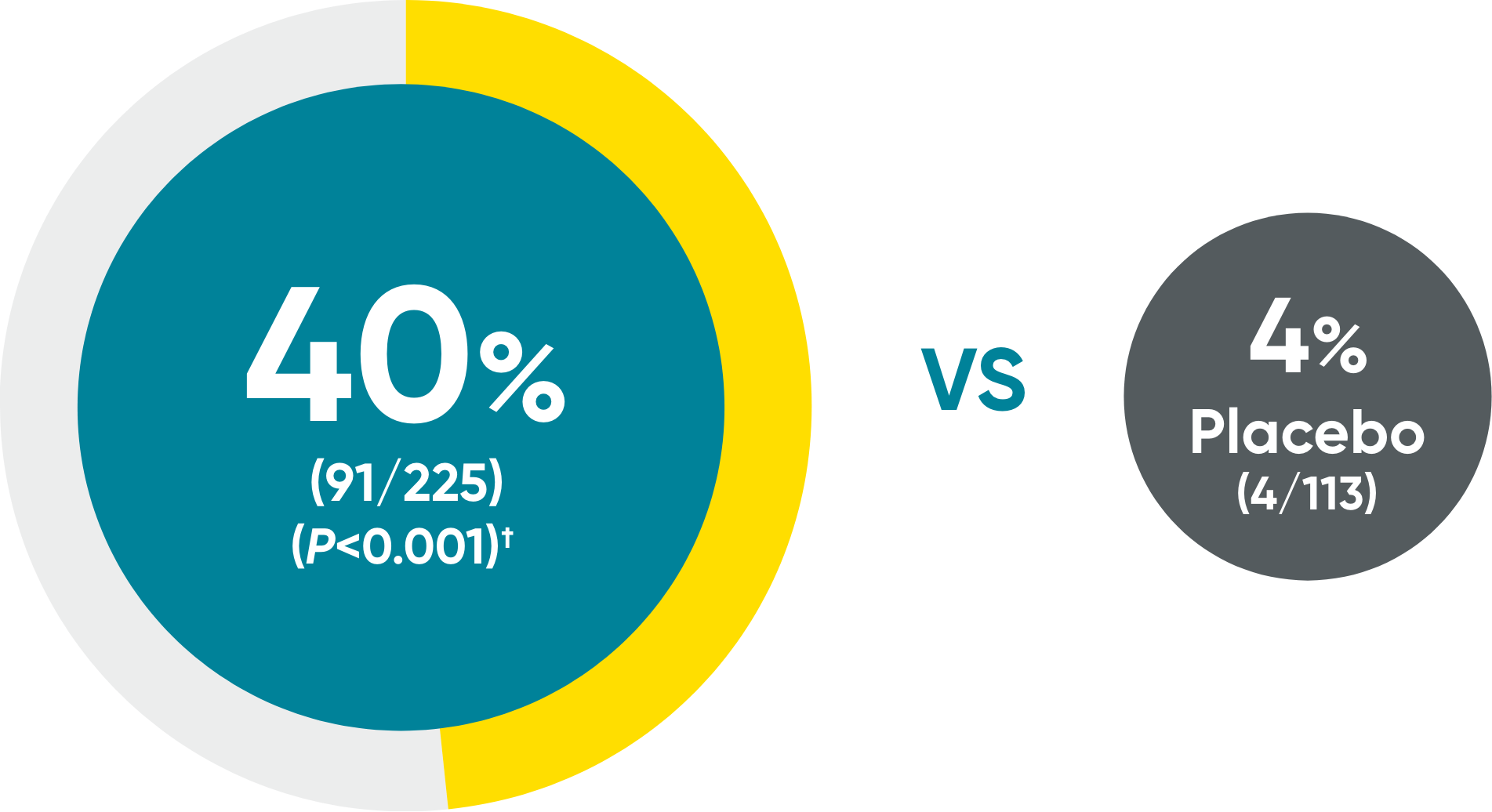
Scalp: 75%
achieved skin clearance (ss-IGA 0/1) with TREMFYA® (114/152)
vs 14% with placebo (11/76) (P<0.001)
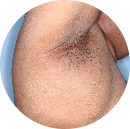
Intertriginous: 86%
achieved skin clearance (i-IGA O/1) with TREMFYA® (96/111)
vs 29% with placebo (15/52) (P<0.001)

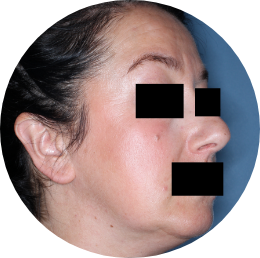
Face: 88%
achieved skin clearance (f-IGA 0/1) with TREMFYA® (79/90)
vs 29% with placebo (12/42) (P<0.001)
Image
is cross polarized.
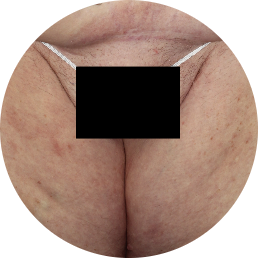
Genital: 78%
achieved skin clearance (sPGA-G) with TREMFYA® (64/82)
vs 38% with placebo (15/40) (P<0.001)


Scalp: 75%
achieved skin clearance (ss-IGA 0/1) with TREMFYA® (114/152)
vs 14% with placebo (11/76) (P<0.001)

Face: 88%
achieved skin clearance (f-IGA 0/1) with TREMFYA® (79/90)
vs 29% with placebo (12/42) (P<0.001)

Intertriginous: 86%
achieved skin clearance (i-IGA O/1) with TREMFYA® (96/111)
vs 29% with placebo (15/52) (P<0.001)

Image
is cross polarized.
Genital: 78%
achieved skin clearance (sPGA-G) with TREMFYA® (64/82)
vs 38% with placebo (15/40) (P<0.001)
Actual patients from the SPECTREM study. Individual results may vary.
Images are Janssen-owned from blinded trial: NCT06039189.
VOYAGE 1 and VOYAGE 2 co-primary endpoints at Week 16 (NRI)5-7:
VOYAGE 1—PASI 90: TREMFYA® 73% (241/329), placebo 3% (5/174) (P<0.001). IGA 0/1: TREMFYA® 85% (280/329),
placebo 7% (12/174) (P<0.001).
VOYAGE 2—PASI 90: TREMFYA® 70% (347/496), placebo 2% (6/248) (P<0.001). IGA 0/1: TREMFYA® 84% (417/496), placebo 8% (21/248) (P<0.001).
VOYAGE 1 and VOYAGE 2: Major secondary endpoint at Week 16 (NRI)5-7:
VOYAGE 1—ss-IGA 0/1 and ≥2-grade improvement from baseline: TREMFYA® 83% (231/277), placebo 15% (21/145) (P<0.001)
VOYAGE 2—ss-IGA 0/1 and ≥2-grade improvement from baseline: TREMFYA® 81% (329/408), placebo 11% (22/202) (P<0.001)
An improvement was seen in psoriasis involving the scalp in subjects randomized to TREMFYA® compared to placebo at Week 16.
*BSA criteria of 2%-15%.
†P value is based on the chi-squared test, not adjusted for baseline stratification factor.
BSA=body surface area; f-IGA=facial-IGA;
IGA=Investigator’s Global Assessment;
i-IGA=intertriginous-IGA; NRI=nonresponder imputation;
sPGA-G=static Physician’s
Global Assessment-genital;
ss-IGA=scalp-specific IGA.
References: 1. Horner ME, Orroth KK, Ma J, et al. Redefining disease severity with special area involvement and reflecting on treatment patterns in a real-world psoriasis population. Dermatol Ther. 2024;14:187-199. 2. Merola JF, Ogdie A, Gottlieb AB, et al. Patient and physician perceptions of psoriatic disease in the United States: results from the UPLIFT survey. Dermatol Ther. 2023;13:1329-1346. 3. Data on file. Janssen Biotech, Inc. 4. Gottlieb Ab, Krueger J, Gordon KB, et al. SPECTREM: Guselkumab demonstrates significant clearance at Week 16 across special sites in participants with low body surface area, moderate psoriasis. Poster presented at: 2024 Fall Clinical Dermatology Conference; October 24-27, 2024; Las Vegas, NV. 5. TREMFYA® (guselkumab) [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc. 6. Blauvelt A, Papp KA, Griffiths CEM, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol.
2017;76(3):405-417. 7. Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol.
2017;76(3):418-431.
IN ADULT PATIENTS WITH MODERATE TO SEVERE PLAQUE PsO


