For US Healthcare Professionals
I am a:

The TREMFYA® 8-week dosing schedule was optimized in clinical trials1,2

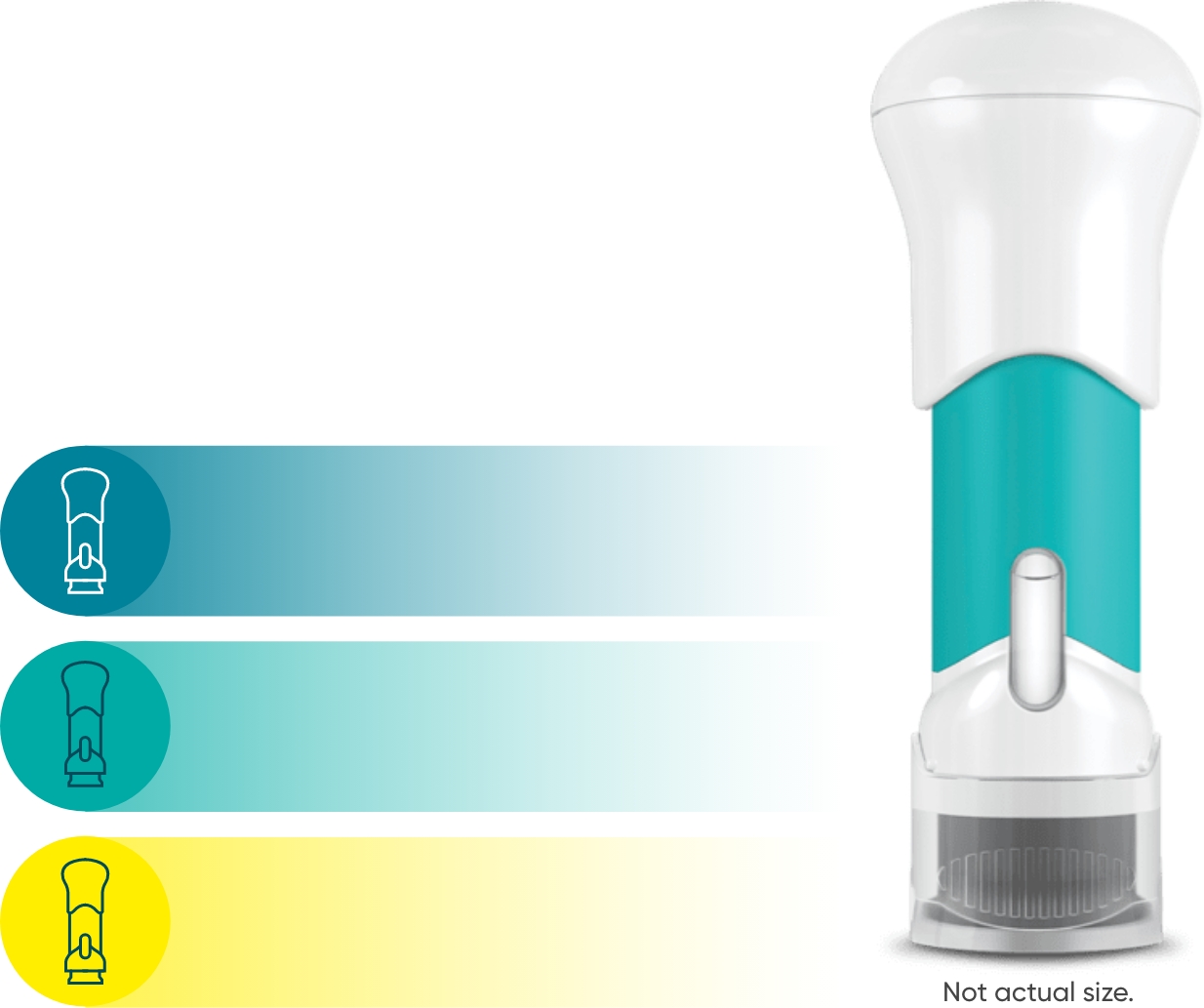

One 100-mg injection
on day 1
Then 1 injection
4 weeks later
Then 1 injection
every 8 weeks thereafter
Available in 2 dosage forms
100 mg/mL prefilled syringe
(NDC: 57894-640-01)
100 mg/mL One-Press patient-controlled
injector (NDC: 57894-640-11)
Pretreatment Evaluation: Evaluate for tuberculosis (TB) infection; if clinically indicated, evaluate liver enzymes and bilirubin levels. Complete all age-appropriate vaccinations according to current immunization guidelines.
Monitor: For signs and symptoms of active TB during and after treatment with TREMFYA®. If clinically indicated, evaluate liver enzymes and bilirubin levels periodically according to routine patient management.
TREMFYA® is intended for use under the guidance and supervision of a healthcare professional. After proper training in subcutaneous injection technique, adults may self-inject. Pediatric self-administration is not recommended. Administration of TREMFYA® to pediatric patients should be performed by a healthcare provider or by a caregiver who has received training and demonstrated proper subcutaneous injection technique. In active PsA, TREMFYA® can be used alone or in combination with a conventional DMARD (eg, methotrexate).
*Frequency of maintenance injections for TREMFYA® after the first year.
DMARD=disease-modifying antirheumatic drug.
References: 1. TREMFYA® (guselkumab) [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc. 2. Lebwohl M, Langley RG, Zhu Y, et al. Use of dose-exposure-response relationships in Phase 2 and Phase 3 guselkumab studies to optimize dose selection in psoriasis. J Eur Acad Dermatol Venereol. 2019;33(11):2082-2086.
One-Press patient-controlled injector
TREMFYA® is the first and only
anti–IL-23 with a patient-controlled
self-injection device; the patient controls the rate of delivery

Not actual size.
Device features designed with patients in mind
- Can be used in physician's office or at home after physician approval and proper training
- Needle guard keeps needle out of view
- Soft “click” indicates when the injection is complete
- Not made with natural rubber latex

Not actual size.
Device features designed with patients in mind:
- Can be used in physician's office or at home after physician approval and proper training
- Needle guard keeps needle out of view
- Soft “click” indicates when the injection is complete
- Not made with natural rubber latex
Please see the full Instructions for Use (IFU) for TREMFYA® One-Press patient-controlled injector.
IL-23=Interleukin-23.
TREMFYA® is intended for use under the guidance and supervision of a physician. Patients may self-inject with TREMFYA® after physician approval and proper training.
Injection demonstration videos
Watch these easy-to-follow injection demonstration videos for TREMFYA®.
The injection demonstration videos are not meant to replace the IFU that are supplied with TREMFYA®. Instruct patients to read the IFU before using their TREMFYA® prefilled syringe or One-Press injector and each time they get a refill.
TREMFYA® is intended for use under the guidance and supervision of a physician. Patients may self-inject with TREMFYA® after physician approval and proper training.
IN ADULT PATIENTS WITH MODERATE TO SEVERE PLAQUE PsO
