For US Healthcare Professionals
I am a:
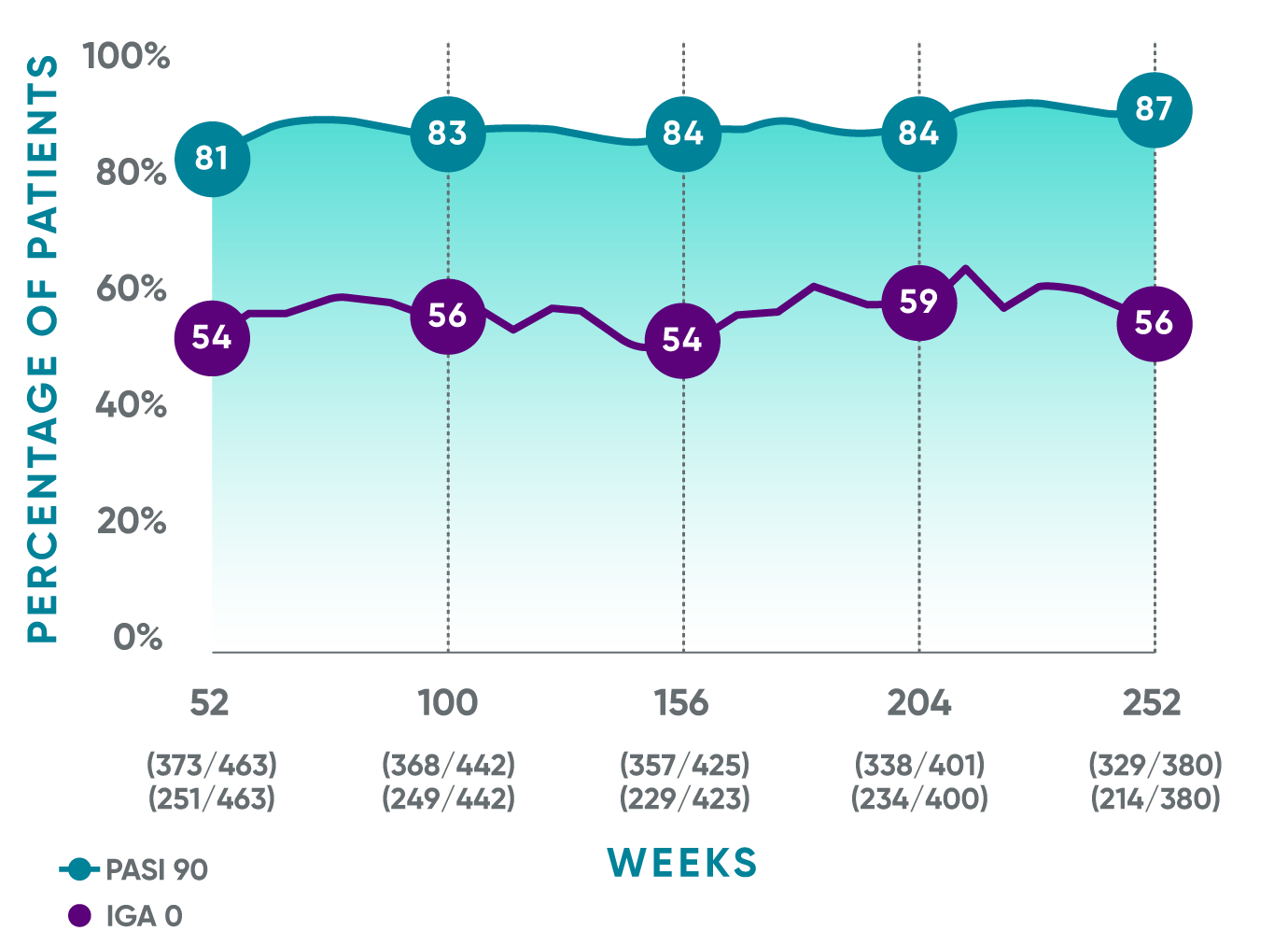
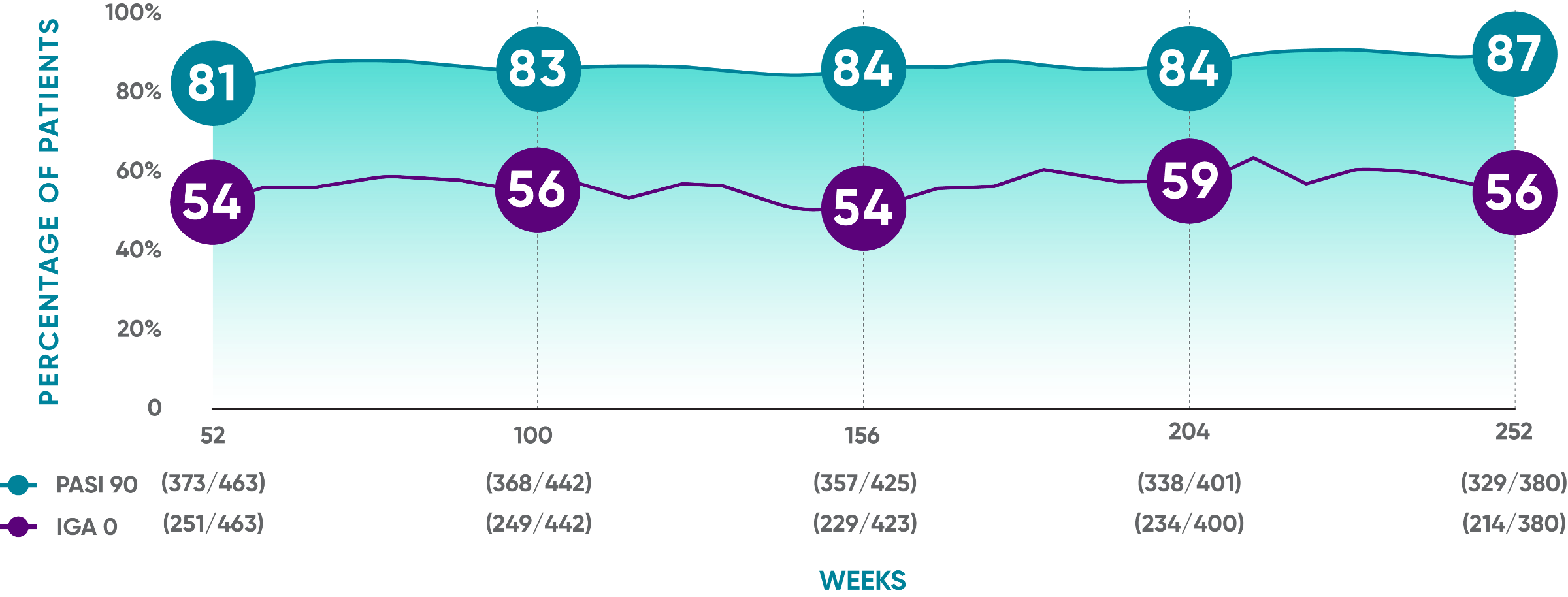
Durable response rates over time1*†
PASI 90 response rates were maintained from Week 52 through Week 252
VOYAGE 1: Prespecified secondary analysis—results through Year 5 (Week 252, as-observed analysis)*†‡


Patients who lose response or are unable to tolerate treatment are likely to discontinue treatment, which may increase the response rate in an as-observed analysis.
*The same patients may not have responded at each time point.
VOYAGE co-primary endpoints at Week 16 (NRI)1,2:
VOYAGE 1—PASI 90: TREMFYA® 73% (241/329), placebo 3% (5/174) (P<0.001). IGA 0/1: TREMFYA® 85% (280/329), placebo 7% (12/174) (P<0.001).1
VOYAGE 2—PASI 90: TREMFYA® 70% (347/496), placebo 2% (6/248) (P<0.001). IGA 0/1: TREMFYA® 84% (417/496), placebo 8% (21/248) (P<0.001).1
In a prespecified secondary analysis of efficacy after Week 48 applying treatment failure rules (TFR)1*†‡:
- PASI 90: 80% (373/468) at Week 52; 82% (368/448) at Week 100; 83% (358/432) at Week 156; 82% (338/411) at Week 204; 84% (329/391) at Week 252
- IGA 0: 54% (251/468) at Week 52; 56% (249/448) at Week 100; 53% (229/430) at Week 156; 57% (234/410) at Week 204; 55% (214/391) at Week 252
VOYAGE 1: PASI 90 and IGA 0 at Week 48 (major secondary endpoints, NRI)2§
- 73% (84/115) of patients receiving TREMFYA® achieved PASI 90, and 47% (54/115) of patients achieved IGA 0
†Available data at each visit were used; missing data were not included in the analysis.
‡Data shown include patients randomized at Week 0 to the TREMFYA® arm and placebo-arm patients who crossed over to receive TREMFYA® at Weeks 16 and 20 and q8w thereafter.
§Results from North American sites only, which used a US-licensed active comparator. Active-comparator data not shown.
References: 1. Data on file. Janssen Biotech, Inc. 2. TREMFYA® (guselkumab) [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc.
IN MODERATE TO SEVERE PLAQUE PSORIASIS (PsO)
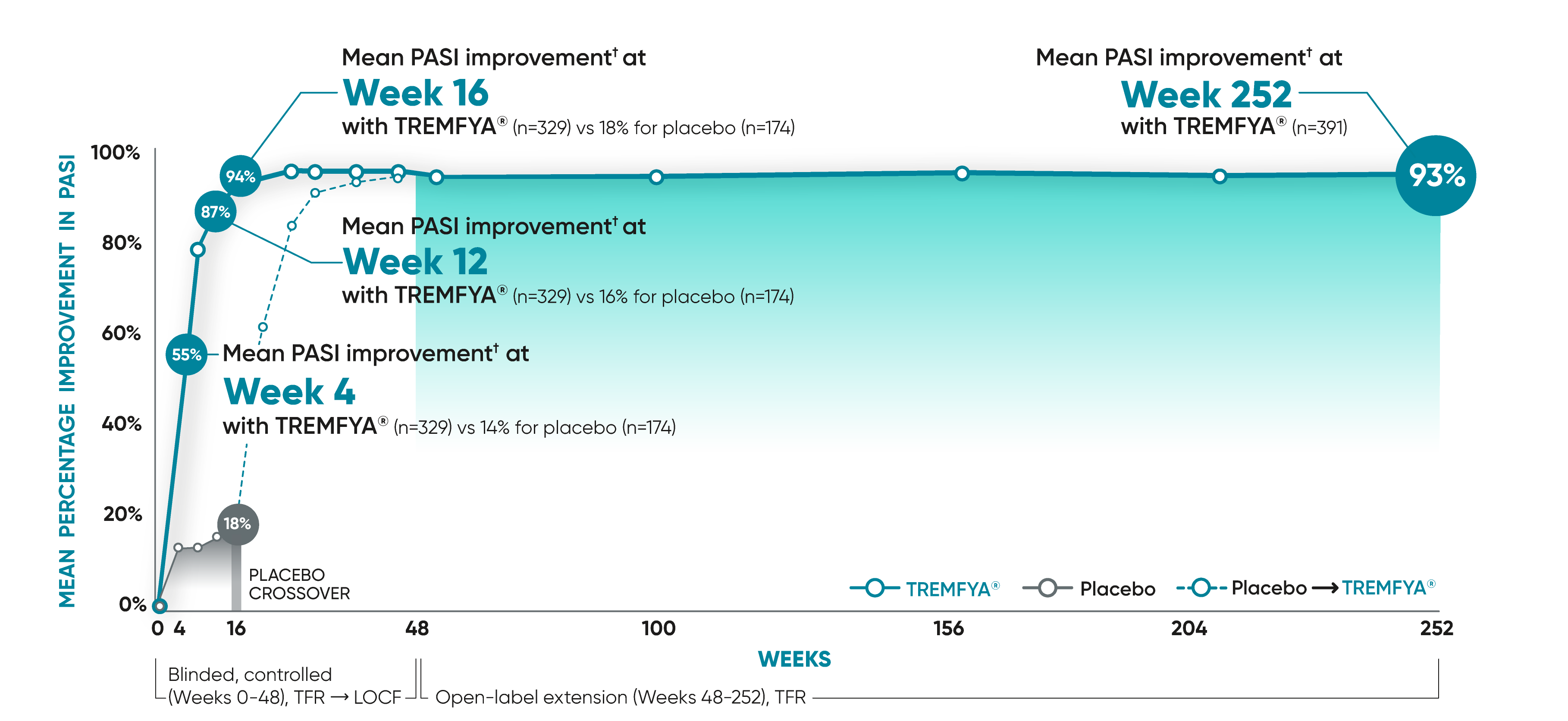
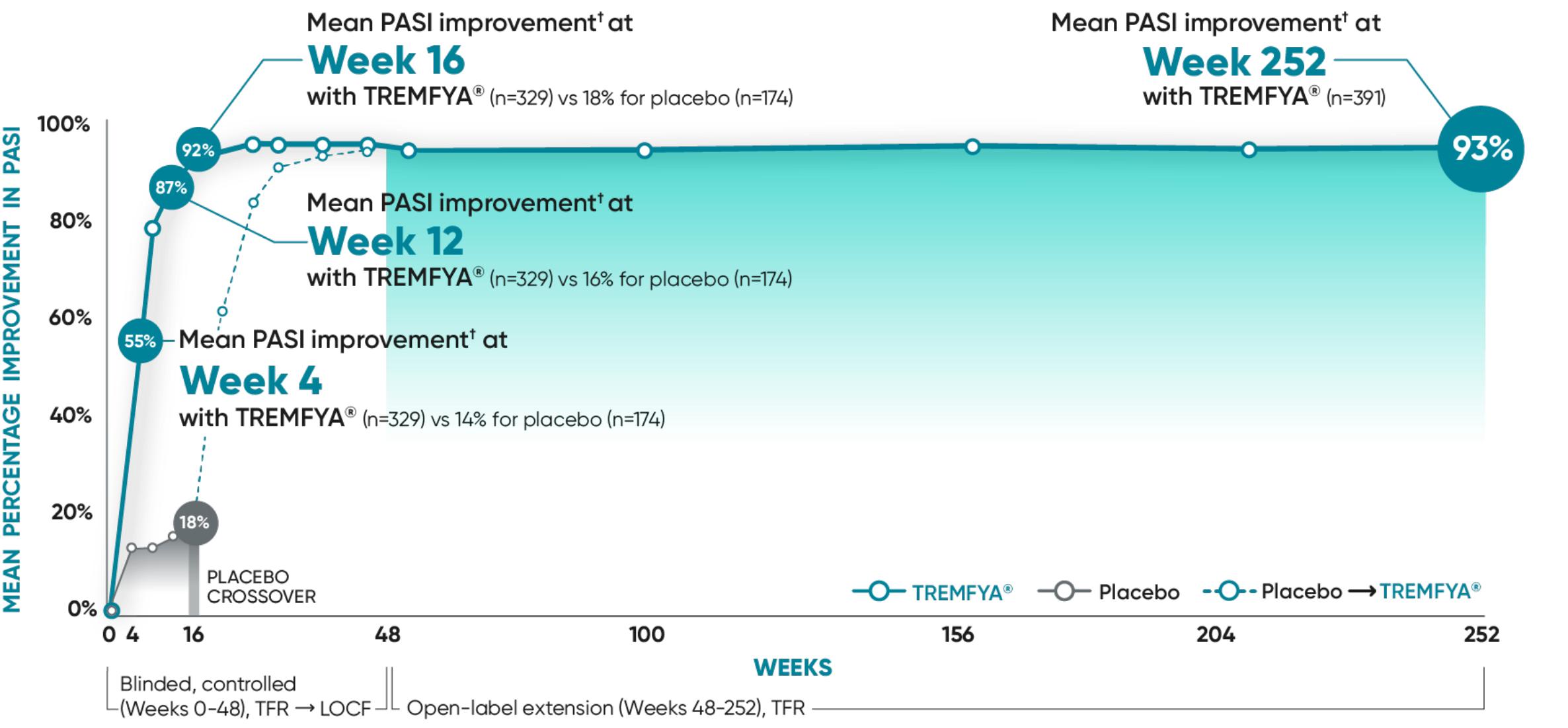
Early results at Week 4 after 1 injection1
VOYAGE 1: Mean PASI improvement from baseline through Year 5 prespecified other secondary analysis (TFR)*†


VOYAGE co-primary endpoints at Week 16 (NRI)1,2:
VOYAGE 1—PASI 90: TREMFYA® 73% (241/329), placebo 3% (5/174) (P<0.001). IGA 0/1: TREMFYA® 85% (280/329), placebo 7% (12/174) (P<0.001).1
VOYAGE 2—PASI 90: TREMFYA® 70% (347/496), placebo 2% (6/248) (P<0.001). IGA 0/1: TREMFYA® 84% (417/496), placebo 8% (21/248) (P<0.001).1
VOYAGE 2: Mean PASI improvement from baseline1
- Week 4: 54% (n=496) with TREMFYA® vs 16% (n=248) with placebo
- Week 16: 89% (n=496) with TREMFYA® vs 20% (n=248) with placebo
In the controlled period, mean PASI improvement was a prespecified other secondary analysis (TFR) that was not adjusted for multiplicity; P values were considered nominal.
Data shown include patients randomized at Week 0 to TREMFYA® arm and placebo arm patients who crossed over to receive TREMFYA® at Weeks 16, 20, and q8w thereafter.
The same patients may not have responded at each time point.
Available data at each visit were used; missing data were not included in the analysis.
TFR methods: Patients who discontinued study agent due to lack of efficacy or an adverse event of worsening of psoriasis, or who started a protocol-prohibited medication, including conventional and biologic systemic therapy, phototherapy, and/or ultra-high-potency corticosteroids were considered treatment failures. Other topical agents for psoriasis were permitted. Patients were considered to have no improvement (percentage improvement=0) after meeting treatment failure criteria.
LOCF=last observation carried forward; PASI=Psoriasis Area and Severity Index; TFR=treatment failure rules.
*Year 5 represents Week 252.
†Mean PASI improvement is an assessment of the average percentage improvement from baseline in psoriatic signs of redness, thickness, scale, and body surface area of involvement.
References: 1. Data on file. Janssen Biotech, Inc. 2. TREMFYA® (guselkumab) [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc.
IN ADULTS WITH MODERATE TO SEVERE PLAQUE PsO


