For US Healthcare Professionals
I am a:
IN ADULT PATIENTS WITH MODERATE TO SEVERE PLAQUE PsO
Established safety profile through 5 years1,2
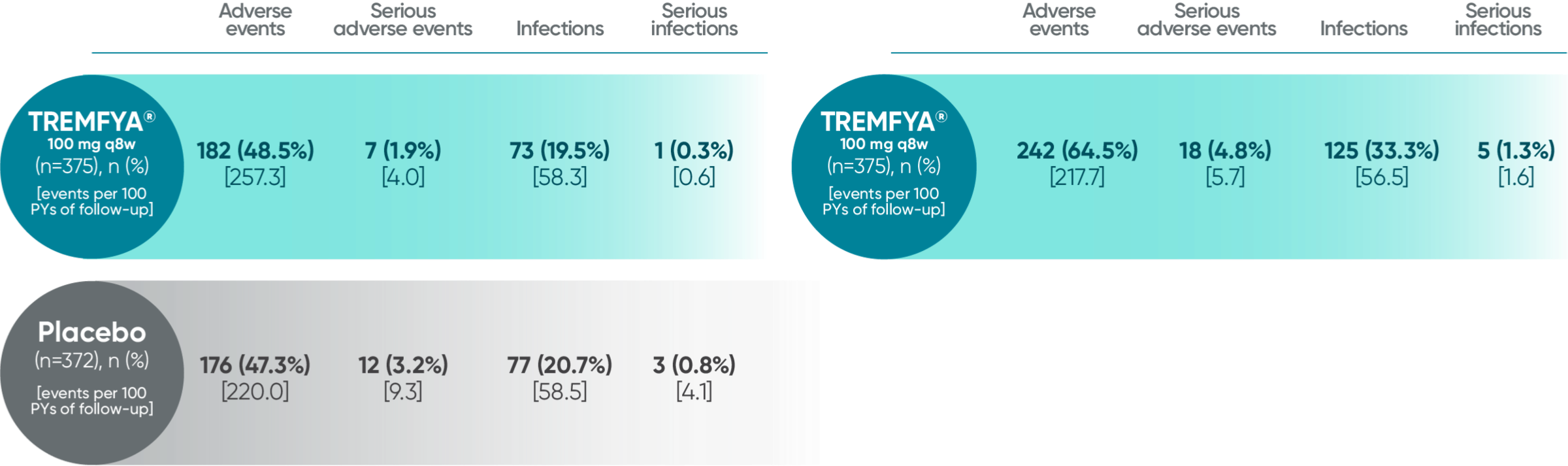
Adverse events in the 16-week, placebo-controlled period of the VOYAGE 1 and VOYAGE 2 pooled clinical trials


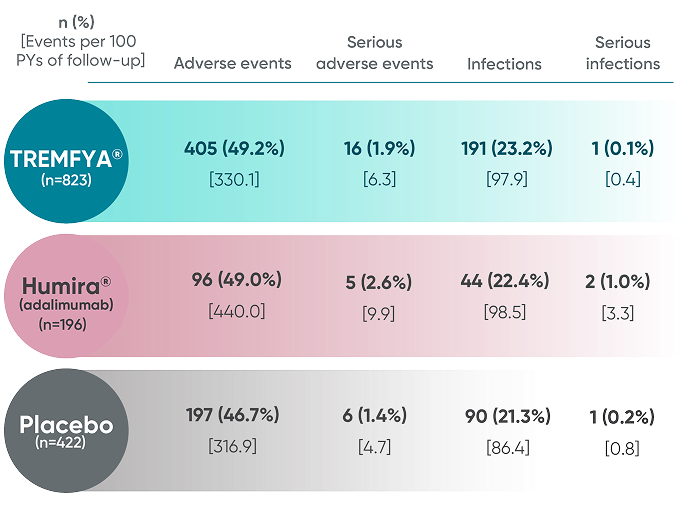

- The most common (≥1%) infections that occurred more frequently in the TREMFYA® group than in the placebo group were upper respiratory infections, gastroenteritis, tinea infections, and herpes simplex infections; all cases were mild to moderate in severity and did not lead to discontinuation of TREMFYA®. The rate of serious infections for the TREMFYA® group and the placebo group was ≤0.2%
Pooled safety data from VOYAGE 1 and VOYAGE 2 through 5 years (Week 264)1,2*


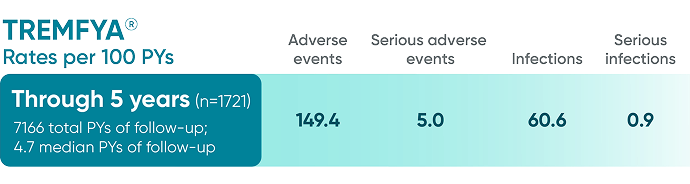

BSA=body surface area; PsA=psoriatic arthritis; PsO=psoriasis; PYs=patient-years.
*Safety summary includes all patients exposed to TREMFYA®.
Humira is a registered trademark of Abbvie Biotechnology Ltd. Corporation.
References: 1. TREMFYA® (guselkumab) [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc. 2. Data on file. Janssen Biotech, Inc.
IN ADULT PATIENTS WITH MODERATE TO SEVERE PLAQUE PSORIASIS (PsO)
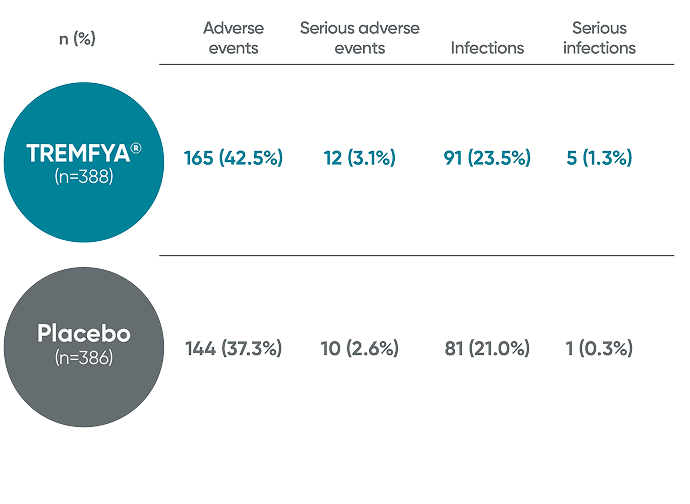
VISIBLE safety data were consistent with pivotal trials1,2
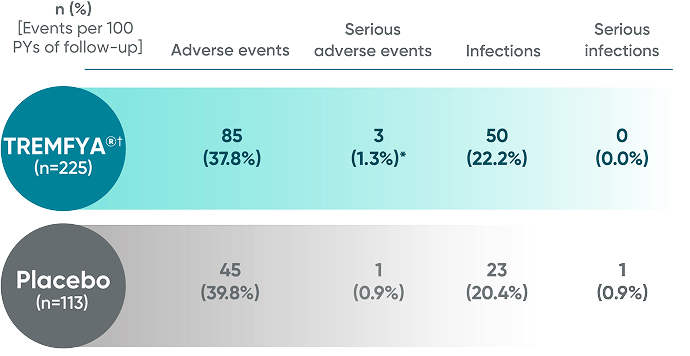
VISIBLE trial: Adverse events in the 16-week, placebo-controlled period (pooled data from Cohorts A and B)1*




No new safety signals were reported in VISIBLE through Week 48
PYs=patient-years.
*Study is blinded and currently ongoing.
†Patients received 100 mg TREMFYA® at Week 0, Week 4, and every 8 weeks thereafter.
References: 1. Data on file. Janssen Biotech, Inc. 2. TREMFYA® (guselkumab) [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc.
IN ADULT PATIENTS WITH MODERATE PLAQUE PSORIASIS
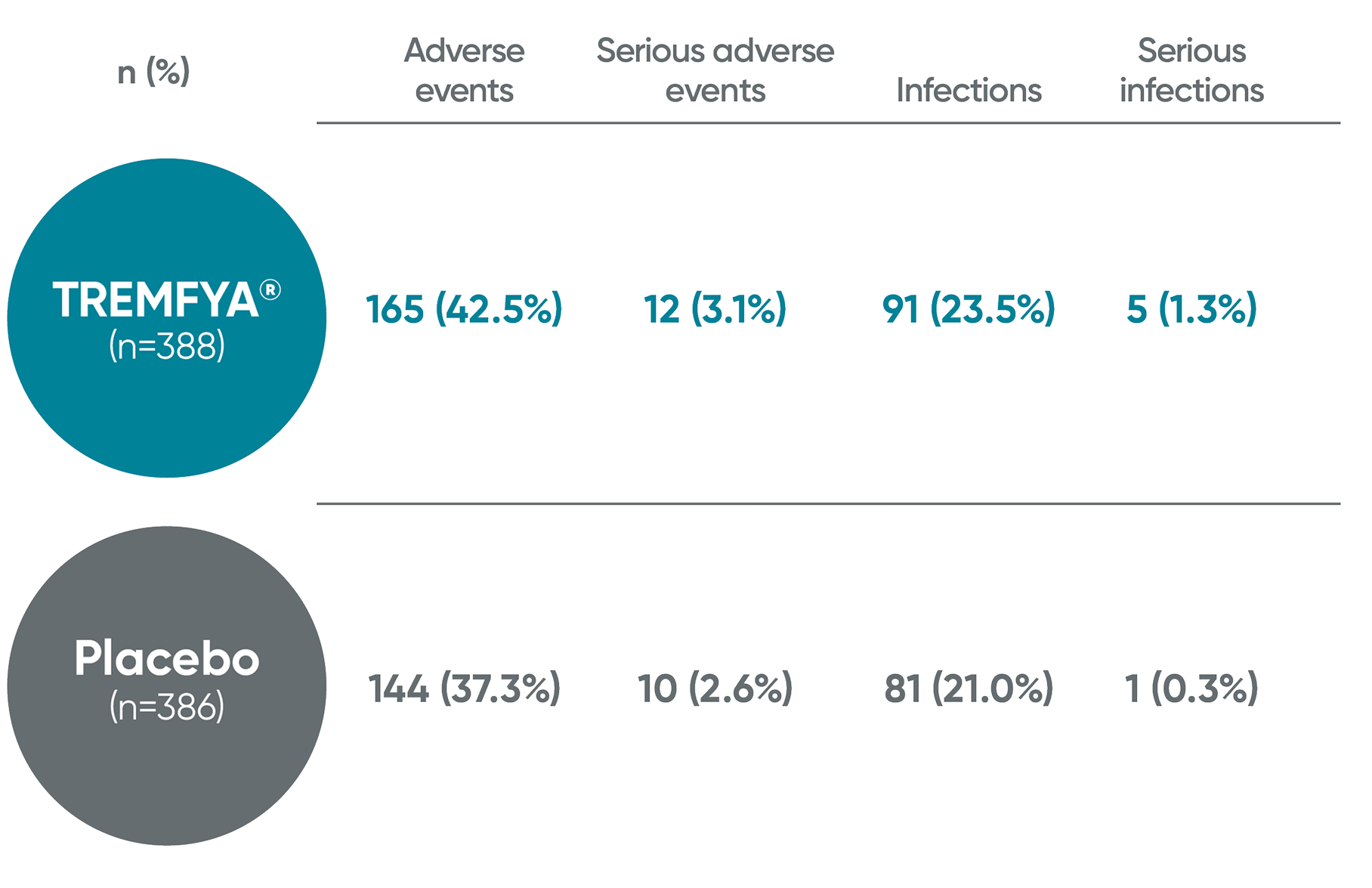
SPECTREM safety profile1,2
SPECTREM adverse events in the 16-week period


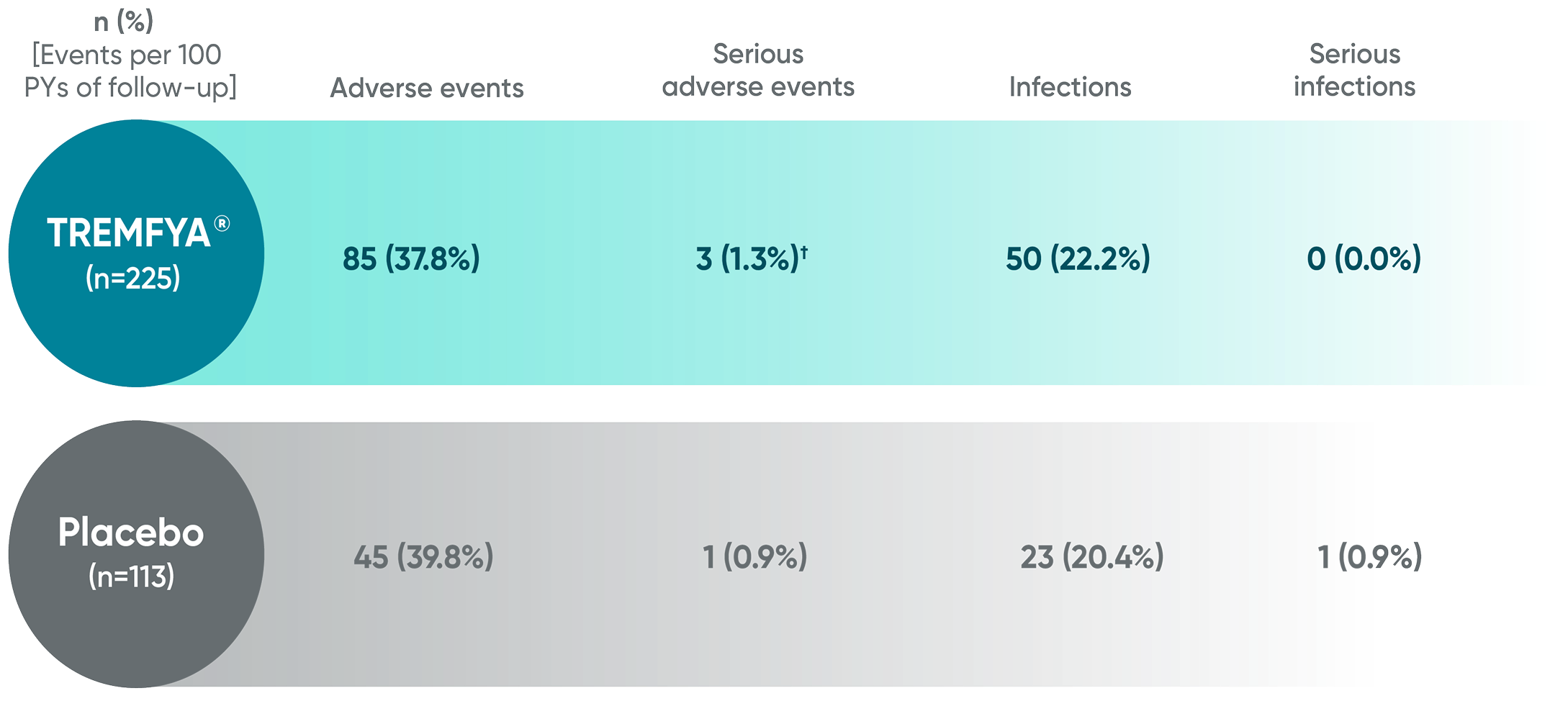
No new safety concerns identified through Week 161†
PYs=patient-years.
*One event each of upper limb fracture, renal colic, and cerebrovascular accident.
†Study is blinded and currently ongoing.
References: 1. Data on file. Janssen Biotech, Inc. 2. Stein Gold L, Strober B, Armstrong AW, et al. SPECTREM: Guselkumab demonstrates consistent significant clearance at week 16 across the full range of low body surface area, moderate psoriasis with special site involvement. Poster presented at: 2024 Fall Clinical Dermatology Conference; October 24-27; Las Vegas, NV.
IN ADULT PATIENTS WITH ACTIVE PsA
Proven safety
profile through 2 years1*
Adverse events reported in the placebo-controlled phase through Week 24 combined across DISCOVER 1 and DISCOVER 2
Adverse events reported through Year 1† combined across DISCOVER 1 and DISCOVER 2



Adverse events reported through Year 1† combined across DISCOVER 1 and DISCOVER 2



In the 24-week, placebo-controlled period of the combined DISCOVER 1 and DISCOVER 2 clinical trials2
- The overall safety profile observed in patients with active PsA treated with TREMFYA® is generally consistent with the profile in patients with plaque PsO, with the addition of bronchitis (occurred in 1.6% and 1.1% of patients in the TREMFYA® q8w group and placebo group, respectively) and neutrophil count decreased (occurred in 0.3% and 0% of patients in the TREMFYA® q8w group and placebo group, respectively)2:
- —The majority of events of neutrophil count decreased were mild, transient, not associated with infection, and did not lead to discontinuation
Adverse events reported through end of study (112 weeks) in DISCOVER 2 only2




PsA=psoriatic arthritis; PsO=psoriasis; PYs=patient-years; q8w=every 8 weeks.
*Through Week 112 in DISCOVER 2.
†1 Year is defined as 60 weeks (through end of study) in DISCOVER 1 and 52 weeks in DISCOVER 2.
References: 1. Data on file. Janssen Biotech, Inc.
2. TREMFYA® (guselkumab) [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc.
IN ADULT PATIENTS WITH ACTIVE PsA
APEX: Proven safety profile through Week 241,2
Adverse events reported in the placebo-controlled phase through Week 24 in APEX
References: 1. Mease PJ, Ritchlin CT, Coates LC, et al. Inhibition of structural damage progression with guselkumab, a selective IL-23i, in participants with active PsA: Results through Week 24 of the phase 3b, randomized, double-blind, placebo-controlled APEX study. Oral presentation at: European Alliance of Associations for Rheumatology (EULAR) 2025 Congress; June 11-14, 2025; Barcelona, Spain. 2. Mease PJ, Ritchlin CT, Coates LC, et al. Inhibition of structural damage progression with guselkumab, a selective IL-23i, in participants with active PsA: Results through Week 24 of the phase 3b, randomized, double-blind, placebo-controlled APEX study. Abstract presented at: European Alliance of Associations for Rheumatology (EULAR) 2025 Congress; June 11-14, 2025; Barcelona, Spain. Late-Breaking Abstracts Session II.
IN ADULT PATIENTS WITH MODERATE TO SEVERE PLAQUE PsO AND ACTIVE PsA
Additional safety considerations
TREMFYA® is contraindicated in patients with a history of serious hypersensitivity reaction to guselkumab or any of its excipients. Warnings and precautions include hypersensitivity reactions, infections, tuberculosis (TB), hepatotoxicity, and immunizations. Initially evaluate for TB and monitor patients for signs and symptoms of TB infection during and after treatment.
Most common (≥1%) adverse reactions associated with TREMFYA® include upper respiratory infections, headache, injection site reactions, arthralgia, bronchitis, diarrhea, gastroenteritis, tinea infections, and herpes simplex infections.
No labeled warnings or precautions for
malignancy, exacerbation of inflammatory bowel
disease, candidiasis* or MACE.1
If clinically indicated, evaluate liver enzymes and
bilirubin at baseline, and periodically thereafter
according to routine patient management.1
Established safety profile
based on extensive
patient experience
*Candida infections as adverse reactions occurred in <1% but >0.1% of subjects in the TREMFYA® group and at a higher rate than in the placebo group
through Week 16 in VOYAGE 1 and VOYAGE 2.
MACE=major adverse cardiovascular event (cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke).
Reference: 1. TREMFYA® (guselkumab) [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc.
IN ADULT PATIENTS WITH MODERATE TO SEVERE PLAQUE PsO


