For US Healthcare Professionals
I am a:
Minority
representation has
been less than ~30% in PsO biologic trials2



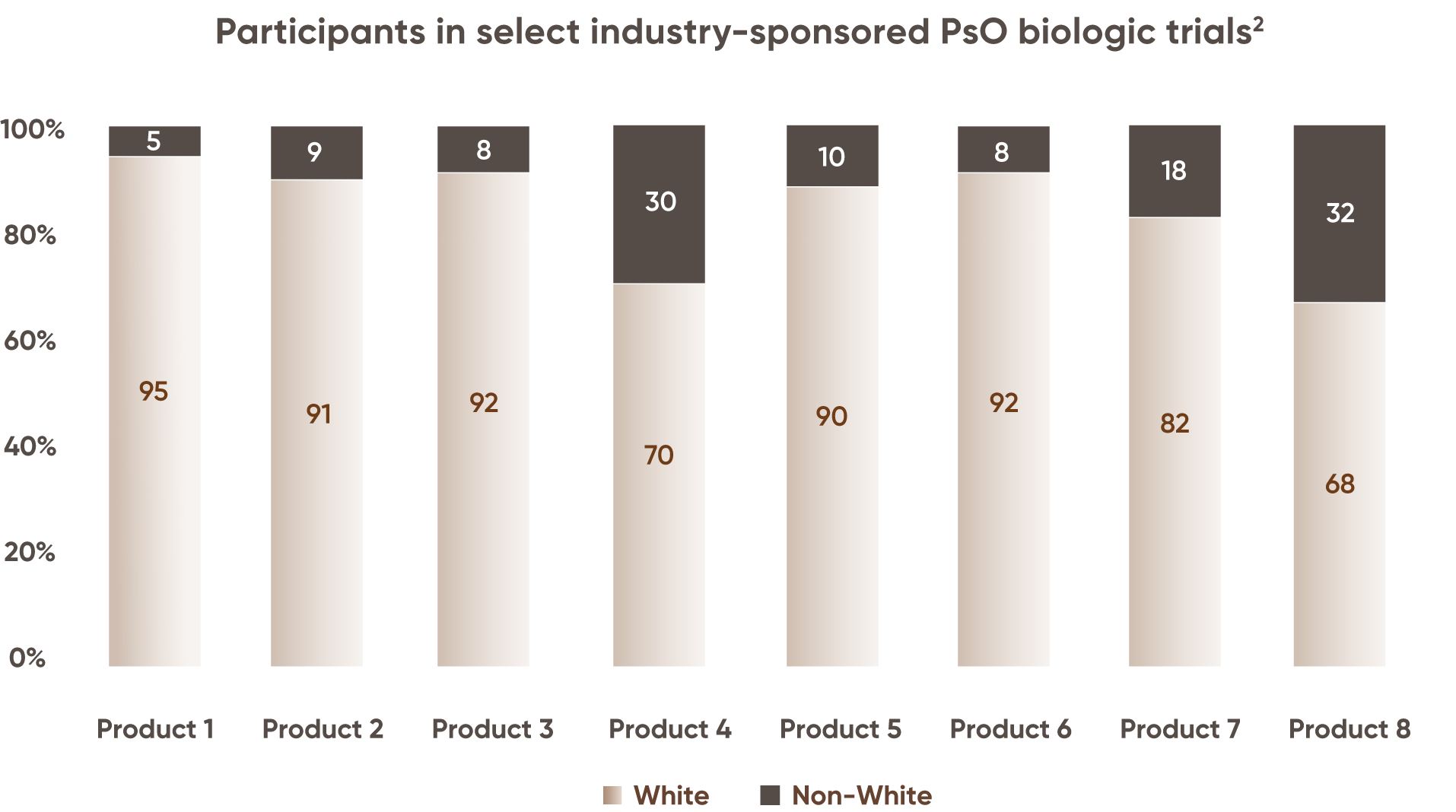
disease prevalence of PsO in non-Hispanic White patients
Underrepresentation in clinical trials may help to explain challenges facing patients with skin of color.
Factors that may impact patients with skin of color
Wait 3x
longer
for final PsO
diagnosis4
4x more
likely
to require a
biopsy to confirm
PsO diagnosis4
Dark skin
ranged from
4%-19%
of images in
dermatology
textbooks5-7
Further investment in research and clinical study efforts will be key to closing these education gaps and may improve diagnosis and care for patients of color.
References: 1. Data on file. Janssen Biotech, Inc. 2. Kaufman BP, Alexis AF. Psoriasis in skin of color: insights into the epidemiology, clinical presentation, genetics, quality-of-life impact, and treatment of psoriasis in non-white racial/ethnic groups. Am J Clin Dermatol. 2018;19(3):405-423. 3. Armstrong AW, Mehta MD, Schupp CW, et al. Psoriasis prevalence in adults in the United States. JAMA Dermatol. 2021;157(8):940-946. 4. Dickerson T, Pratt A, O’Quinn M, et al. Racial disparities in the diagnosis of psoriasis. Cutis. 2022;110:26-28. 5. Wilson BN, Sun M, Ashbaugh AG, et al. Assessment of skin of color and diversity and inclusion content of dermatologic published literature: an analysis and call to action. Int J Women’s Derm. 2021;7:391-397. 6. Ebede T, Papier A. Disparities in dermatology educational resources. J Am Acad Dermatol. 2006;55(4):687-690. 7. Adelekun A, Onyekaba G, Lipoff JB. Skin color in dermatology textbooks: an updated evaluation and analysis. J Am Acad Dermatol. 2021;84(1):194-196.
IN MODERATE TO SEVERE PLAQUE PSORIASIS (PsO)
Skin clearance across all skin tones* at Weeks 16 and 481,2
Fitzpatrick VI
Week 0


PASI=45.1
Week 16


PASI=9.6
(79% PASI
improvement)
Week 48


PASI=3.30
(93% PASI
improvement)
Actual patient from the VISIBLE study.
Individual results may vary.
Images are Janssen-owned from blinded trial: NCT05272150.
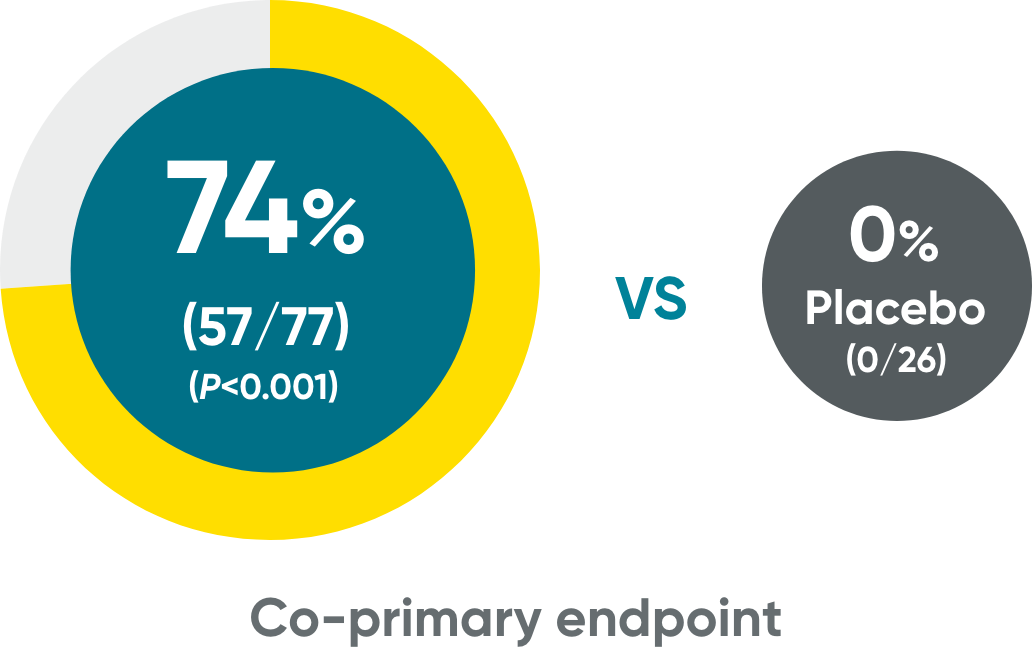
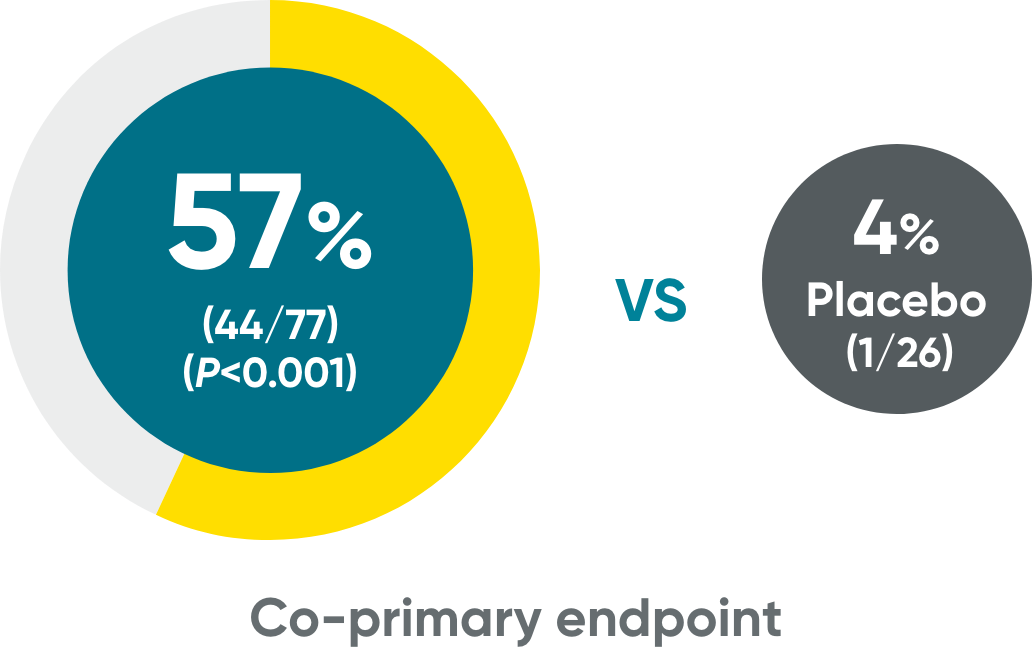
IGA 0/1 and PASI 90 at Week 16 (NRI)
IGA 0/1†
PASI 90†
IGA 0/1 and PASI 90 at Week 48 (NRI)
IGA 0/1‡
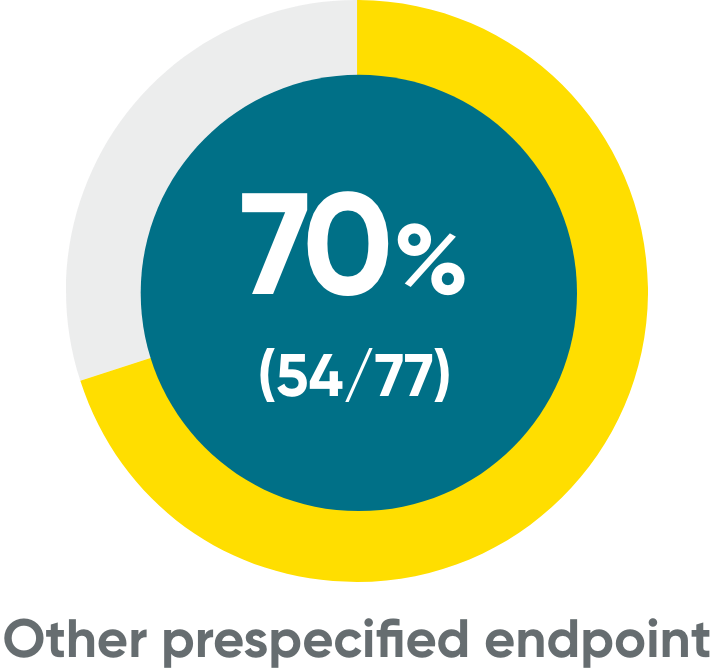
PASI 90‡

Week 48 data were prespecified and not multiplicity controlled.
The same patients may not have responded at each time point.
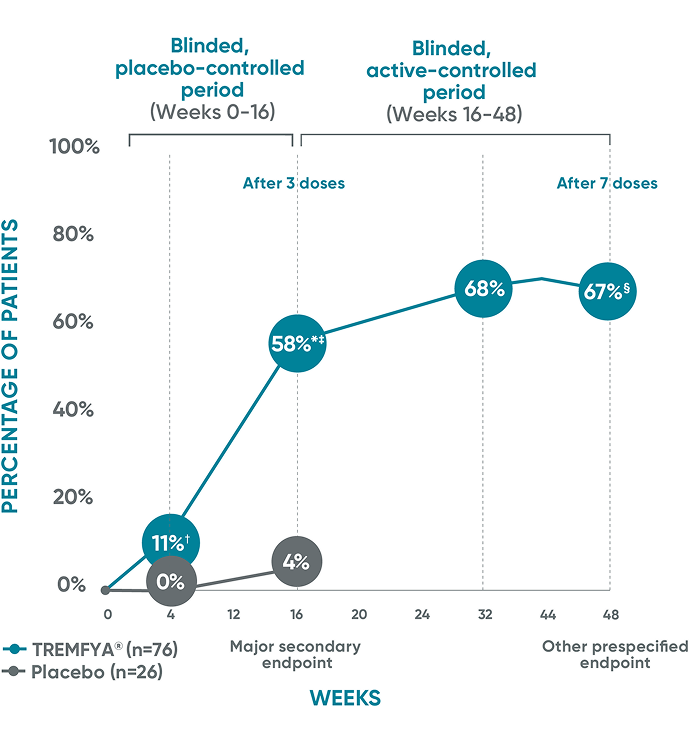
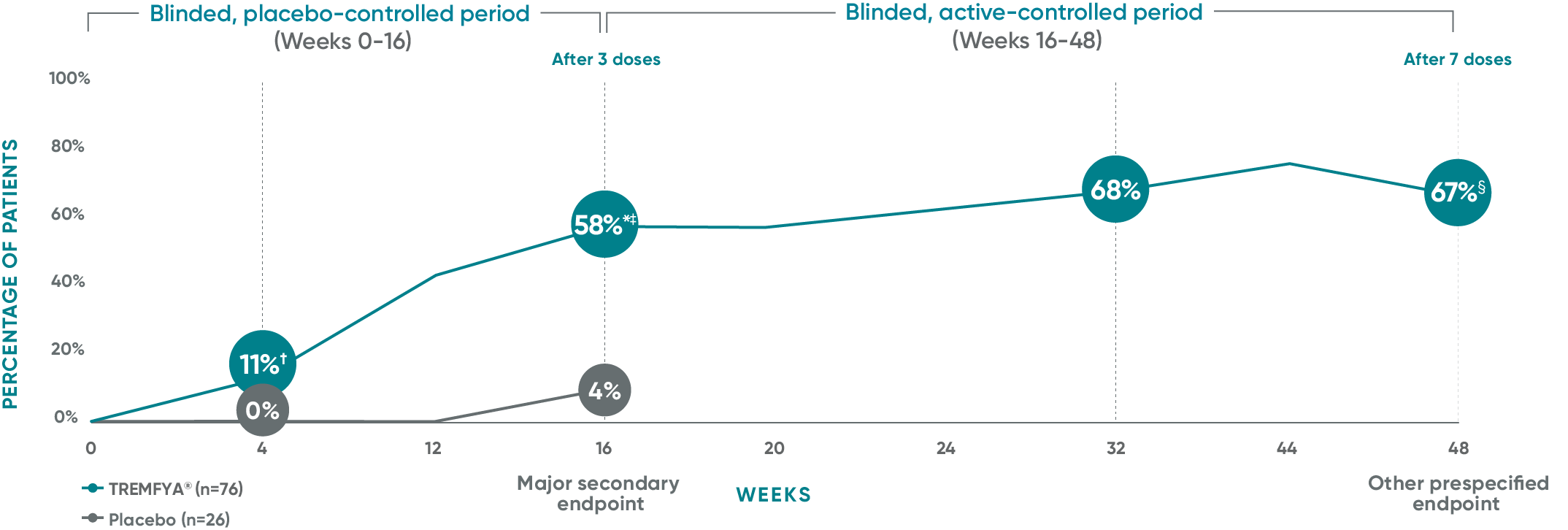
VOYAGE 1 and VOYAGE 2 co-primary endpoints at Week 16 (NRI)3-5:
VOYAGE 1—PASI 90: TREMFYA® 73% (241/329), placebo 3% (5/174) (P<0.001). IGA 0/1: TREMFYA® 85% (280/329), placebo 7% (12/174) (P<0.001). VOYAGE 2—PASI 90: TREMFYA® 70% (347/496), placebo 2% (6/248) (P<0.001). IGA 0/1: TREMFYA® 84% (417/496), placebo 8% (21/248) (P<0.001).
NRI=nonresponder imputation.
*First large-scale, prospective PsO biologic study in patients with skin of color across the entire spectrum of the Fitzpatrick scale (I-VI).
†Week 16 data include patients randomized to TREMFYA® or placebo arms at baseline.
‡Week 48 data include patients randomized to TREMFYA® or placebo arms at baseline. Data for placebo crossover patients are not shown.
IN MODERATE TO SEVERE PLAQUE PsO
Scalp clearance across all skin tones* at Weeks 16 and 481,6
Fitzpatrick III
Week 0


PSSI=60
Week 16
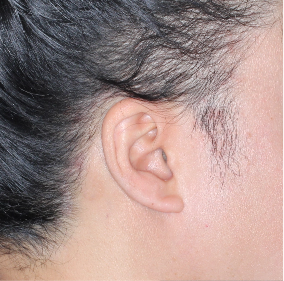

PSSI=6
(90% PSSI
improvement)
Week 48


PSSI=1
(98% PSSI
improvement)
Actual patient from the VISIBLE study.
Individual results may vary.
Images are Janssen-owned from blinded trial: NCT05272150.
ss-IGA 0/1 and PSSI 90 at Weeks 16 (NRI)
ss-IGA 0/1 at Week 16†
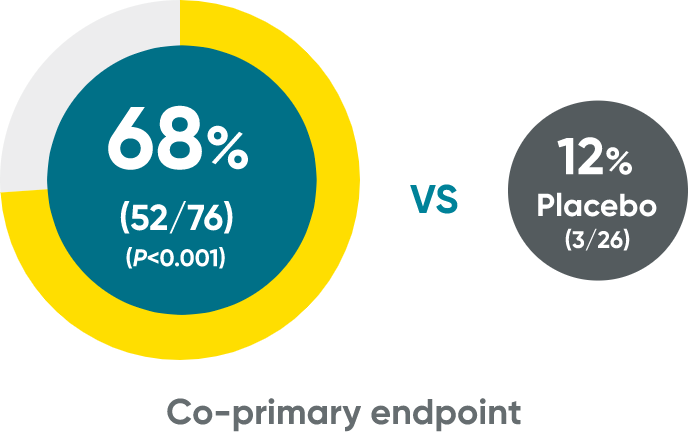
PSSI 90†
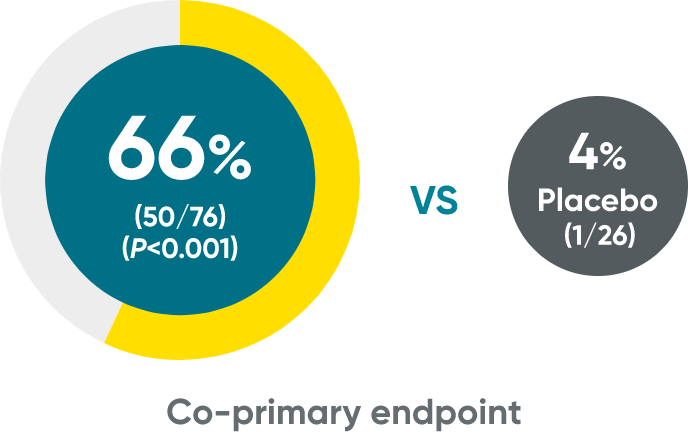
ss-IGA 0/1 and PSSI 90 at Week 48 (NRI)
ss-IGA 0/1‡
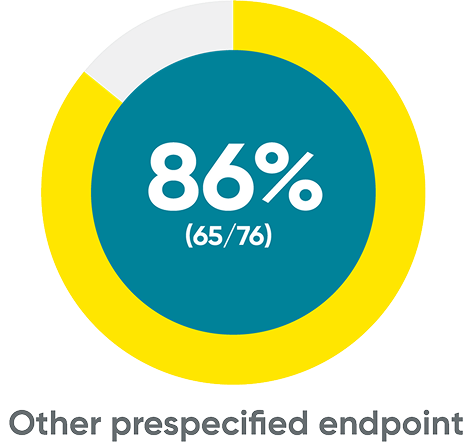
PSSI 90‡
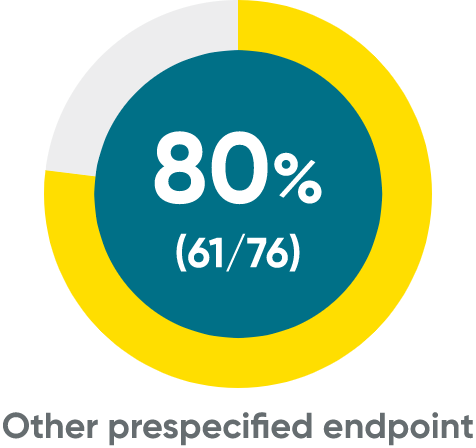
Week 48 data were prespecified and not multiplicity controlled.
The same patients may not have responded at each time point.
In VOYAGE 1 and VOYAGE 2, an improvement was seen in psoriasis involving the scalp in patients randomized to TREMFYA® compared to placebo at Week 16.
NRI=nonresponder imputation; PSSI=Psoriasis Scalp Severity Index; ss-IGA=scalp-specific Investigator’s Global Assessment.
*First large-scale, prospective PsO biologic study in patients with skin of color across the entire spectrum of the Fitzpatrick scale (I-VI).
†Week 16 data include patients randomized to TREMFYA® or placebo arms at baseline.
‡Week 48 data include patients randomized to TREMFYA® at baseline. Data for placebo crossover patients are not shown.
References: 1. Data on file. Janssen Biotech, Inc. 2. Alexis A, et al. Presentation at: Fall Clinical Dermatology Conference; October 19-22, 2023; Las Vegas, NV. 3. TREMFYA® (guselkumab) [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc. 4. Blauvelt A, Papp KA, Griffiths CEM, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator–controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76(3):405-417. 5. Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator–controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76(3):418-431. 6. Alexis A, et al. Presented at: Maui Derm Hawaii; January 22-26, 2024; Maui, HI.
IN MODERATE TO SEVERE PLAQUE PSORIASIS (PsO)
Complete skin clearance rates after 3 doses1,2
Fitzpatrick IV
Week 0


PASI=47.5
Week 16

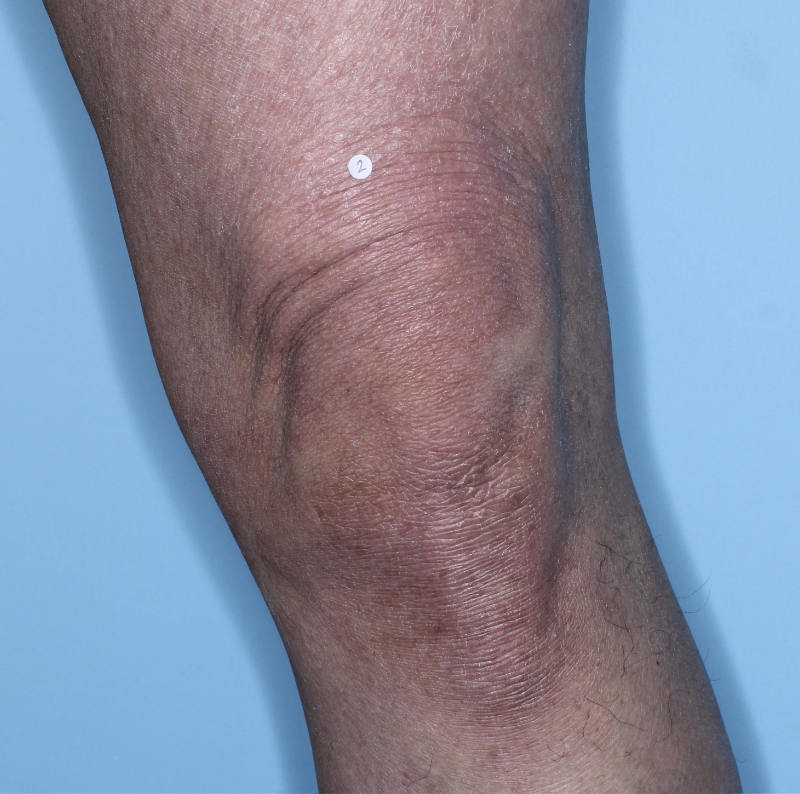
PASI=1.8
(96% PASI
improvement)
Week 48


PASI=0
(100% PASI
improvement)
Actual patient from the VISIBLE study.
Individual results may vary.
Images are Janssen-owned from blinded trial: NCT05272150.




*P<0.002.
Week 48 data were prespecified and not multiplicity controlled.
The same patients may not have responded at each time point.
VOYAGE 1 and VOYAGE 2 co-
primary endpoints at Week 16
(NRI)3-5:
VOYAGE 1—PASI 90: TREMFYA® 73% (241/329), placebo 3% (5/174) (P<0.001). IGA 0/1: TREMFYA® 85% (280/329), placebo 7% (12/174) (P<0.001). VOYAGE 2—PASI 90: TREMFYA® 70% (347/496), placebo 2% (6/248) (P<0.001). IGA 0/1: TREMFYA® 84% (417/496), placebo 8% (21/248) (P<0.001).
In VOYAGE 1 and VOYAGE 2 at Week 24 (major secondary endpoint, NRI)3-5§:
- 53% (61/115) of patients in VOYAGE 1 and 48% (76/160) of patients in VOYAGE 2 achieved IGA 0
NRI=nonresponder imputation.
†Week 16 data include patients randomized to TREMFYA® or placebo arms at baseline.
‡Week 48 data include patients randomized to TREMFYA® at baseline. Data for placebo crossover patients are not shown.
§Results from North American sites only, which used a US-licensed active comparator. Active-comparator data not shown.
IN MODERATE TO SEVERE PLAQUE PSORIASIS (PsO)
Complete scalp skin clearance rates after 3 doses and after 7 doses1,6
Fitzpatrick VI
Week 0


ss-IGA=4
Week 4
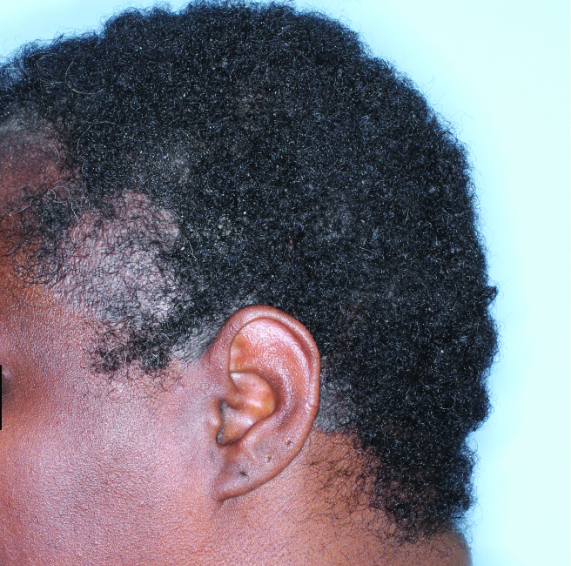
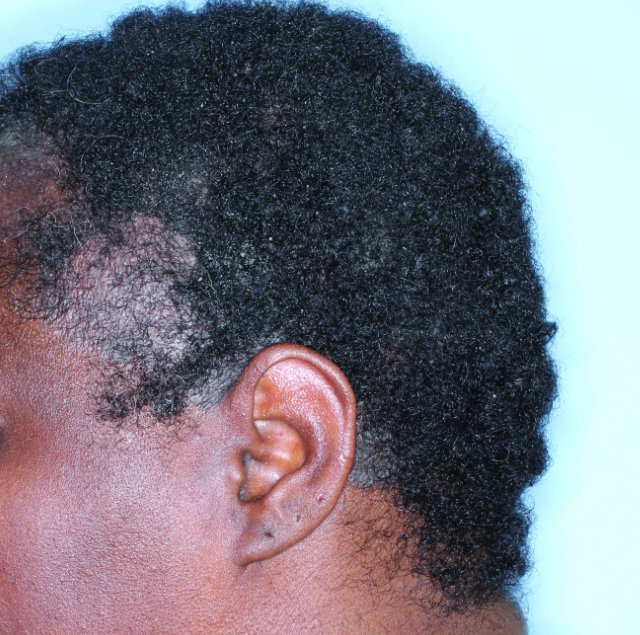
ss-IGA=2
Week 16


ss-IGA=0
Week 48
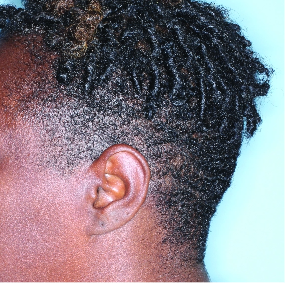

ss-IGA=0
Actual patient from the VISIBLE study.
Individual results may vary.
Images are Janssen-owned from blinded trial: NCT05272150.
ss-IGA 0 at Week 16 and Week 48 (NRI)


Weeks 32 and 48 data were prespecified and not multiplicity controlled.
The same patients may not have responded at each time point.
In VOYAGE 1 and VOYAGE 2, an improvement was seen in psoriasis involving the scalp in patients randomized to TREMFYA® compared to placebo at Week 16.
NRI=nonresponder imputation.
*P<0.001.
†Efficacy measures at Week 4 were prespecified and were not multiplicity controlled. Therefore, P values are nominal and no statistical comparisons can be made.
‡Week 16 data include patients randomized to TREMFYA® or placebo arms at baseline.
§Week 48 data include patients randomized to TREMFYA® at baseline. Data for placebo crossover patients are not shown.
References: 1. Data on file. Janssen Biotech, Inc. 2. Alexis A, et al. Presentation at: Fall Clinical Dermatology Conference; October 19-22, 2023; Las Vegas, NV. 3. TREMFYA® (guselkumab) [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc. 4. Blauvelt A, Papp KA, Griffiths CEM, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator–controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76(3):405-417. 5. Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator–controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76(3):418-431. 6. Alexis A, et al. Presented at: Maui Derm Hawaii; January 22-26, 2024; Maui, HI.
IN MODERATE TO SEVERE PLAQUE PSORIASIS (PsO)
Mean PASI improvement from baseline1
Fitzpatrick III
Week 0

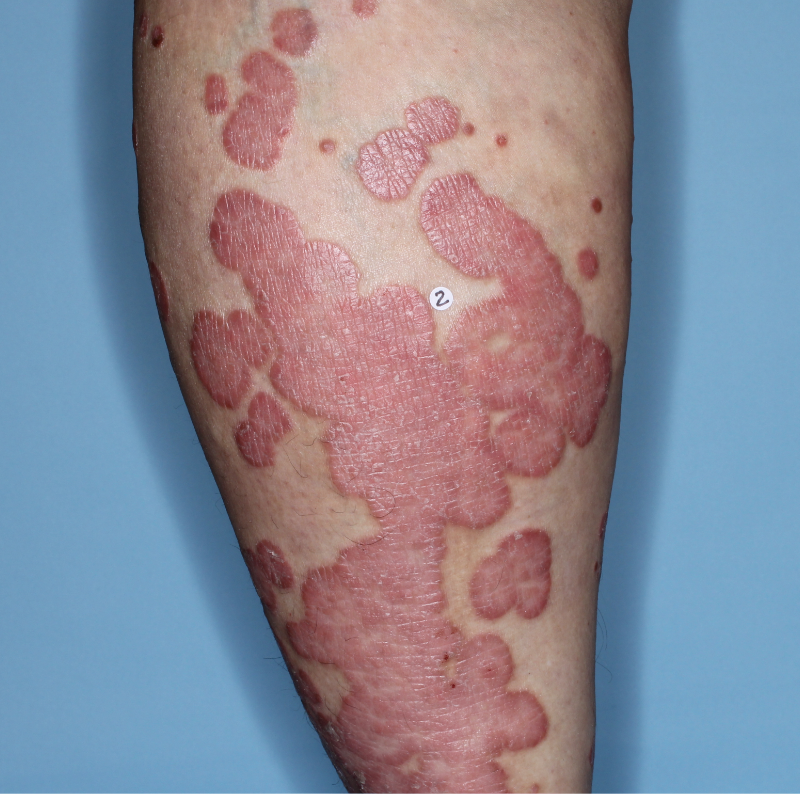
PASI=20.6
Week 16
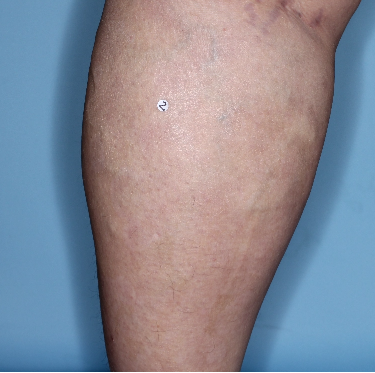
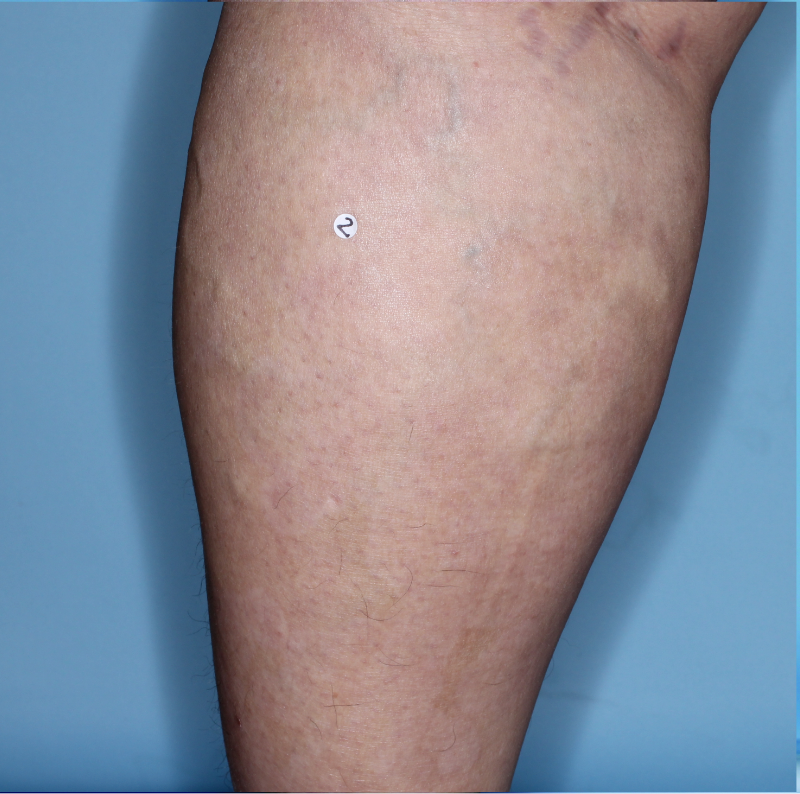
PASI=2.9
(86% PASI
improvement)
Week 48


PASI=1.1
(95% PASI
improvement)
Actual patient from the VISIBLE study.
Individual results may vary.
Images are Janssen-owned from blinded trial: NCT05272150.
Mean PASI improvement from baseline at Week 16 and Week 48*‡§


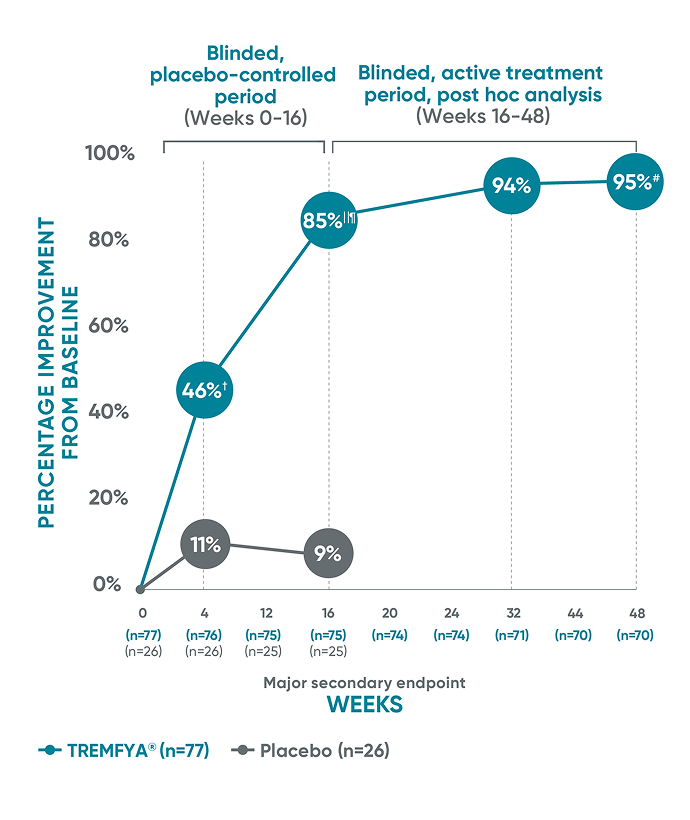

Weeks 16 to 48 are a post hoc analysis; therefore, statistical significance has not been established.
The same patients may not have responded at each time point.
VOYAGE 1 and VOYAGE 2 co-primary endpoints at Week 16 (NRI)2-4:
VOYAGE 1—PASI 90: TREMFYA® 73% (241/329), placebo 3% (5/174) (P<0.001). IGA 0/1: TREMFYA® 85% (280/329), placebo 7% (12/174) (P<0.001).
VOYAGE 2—PASI 90: TREMFYA® 70% (347/496), placebo 2% (6/248) (P<0.001). IGA 0/1: TREMFYA® 84% (417/496), placebo 8% (21/248) (P<0.001).
*Mean PASI improvement is an assessment of the average percentage of improvement from baseline in psoriatic signs of redness, thickness, scale, and body surface area of involvement.
†Efficacy measures at Week 4 were prespecified and were not multiplicity controlled. Therefore, P values are nominal and no statistical comparisons can be made.
‡Patients received 100 mg subcutaneous TREMFYA® at Week 0, Week 4, and every 8 weeks thereafter.
§When participants discontinued study agent due to lack of efficacy, worsening of PsO, or use of a prohibited PsO treatment, zero change from baseline was assigned from that point onward. Missing data were not imputed.
IIWeek 16 data include patients randomized to TREMFYA® or placebo arms at baseline.
¶P<0.001 vs placebo. P values were based on the mixed effect model for repeated measures (MMRM).
#Week 48 data include patients randomized to TREMFYA® at baseline. Data for placebo crossover patients are not shown.
IN MODERATE TO SEVERE PLAQUE PSORIASIS (PsO)
Mean BSA improvement from baseline1
Fitzpatrick VI
Week 0
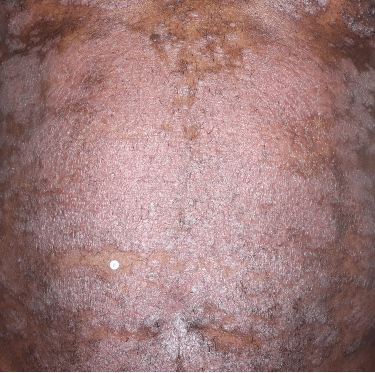
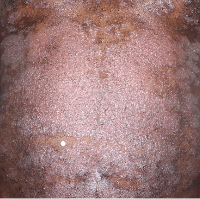
BSA=75%
Week 16
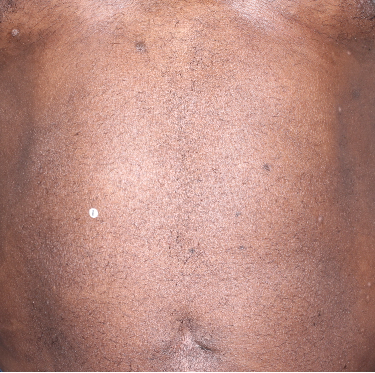
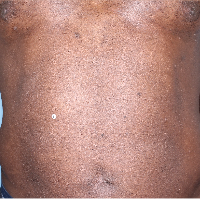
BSA=14%
(81.3% BSA
improvement)
Week 48
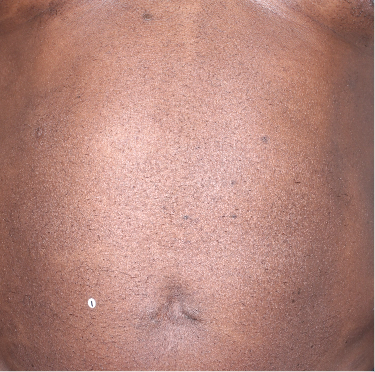

BSA=4%
(94.7% BSA
improvement)
Actual patient from the VISIBLE study.
Individual results may vary.
Images are Janssen-owned from blinded trial: NCT05272150.
Mean BSA improvement from baseline at Week 16 and Week 48*†
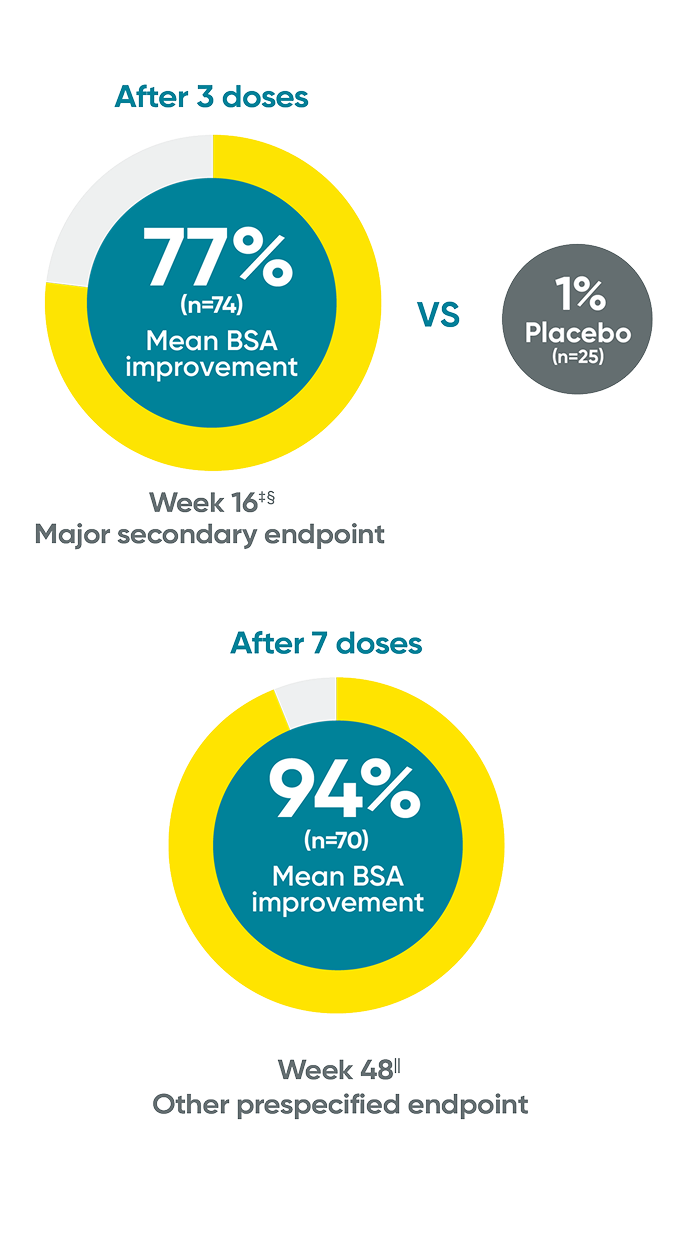
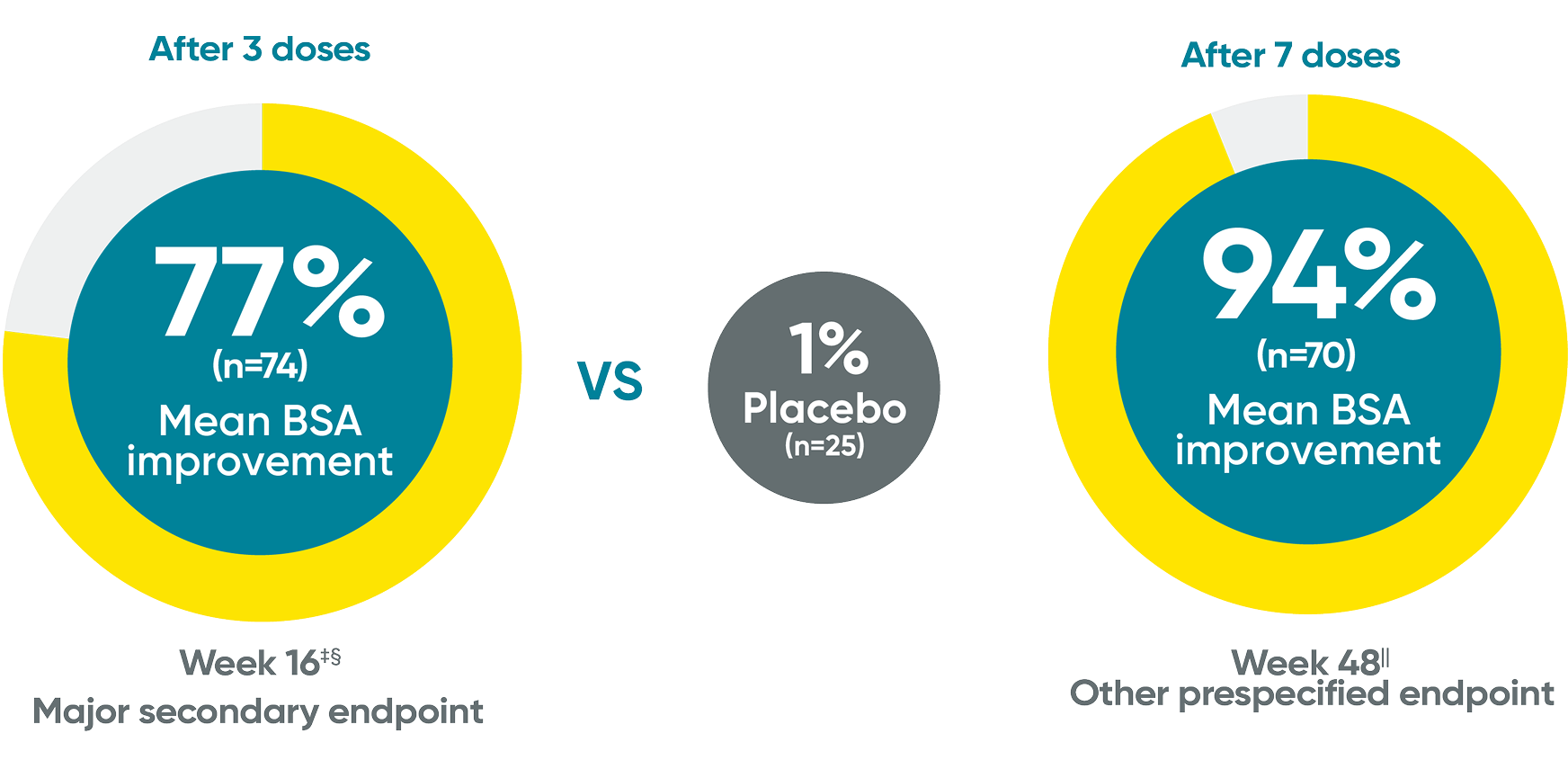
Week 48 data were prespecified and not multiplicity controlled.
The same patients may not have responded at each time point.
VOYAGE 1 and VOYAGE 2 co-primary endpoints at Week 16 (NRI)2-4:
VOYAGE 1—PASI 90: TREMFYA® 73% (241/329), placebo 3% (5/174) (P<0.001). IGA 0/1: TREMFYA® 85% (280/329), placebo 7% (12/174) (P<0.001). VOYAGE 2—PASI 90: TREMFYA® 70% (347/496), placebo 2% (6/248) (P<0.001). IGA 0/1: TREMFYA® 84% (417/496), placebo 8% (21/248) (P<0.001).
*Mean body surface area (BSA) improvement is an assessment of the average percentage improvement from baseline in the BSA of involvement.
†When participants discontinued study agent due to lack of efficacy, worsening of PsO, or use of a prohibited PsO treatment, zero change from baseline was assigned from that point onward. Missing data were not imputed.
‡Week 16 data include patients randomized to TREMFYA® or placebo arms at baseline.
§P<0.001 vs placebo. P values were based on the mixed-effect model for repeated measures (MMRM).
IIWeek 48 data include patients randomized to TREMFYA® at baseline. Data for placebo crossover patients are not shown.
References: 1. Data on file. Janssen Biotech, Inc. 2. TREMFYA® (guselkumab) [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc. 3. Blauvelt A, Papp KA, Griffiths CEM, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator–controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76(3):405-417. 4. Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator–controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76(3):418-431.
IN ADULT PATIENTS WITH MODERATE TO SEVERE PLAQUE PsO


