For US Healthcare Professionals
I am a:
® vs Cosentyx® (secukinumab)
ECLIPSE study design
ECLIPSE: Phase 3, double-blind trial (n=1048)1,2




As of May 2023, Cosentyx® is available in a 150 mg/mL and a 300 mg/2 mL single-dose injection.
Cosentyx is a registered trademark of Novartis AG.
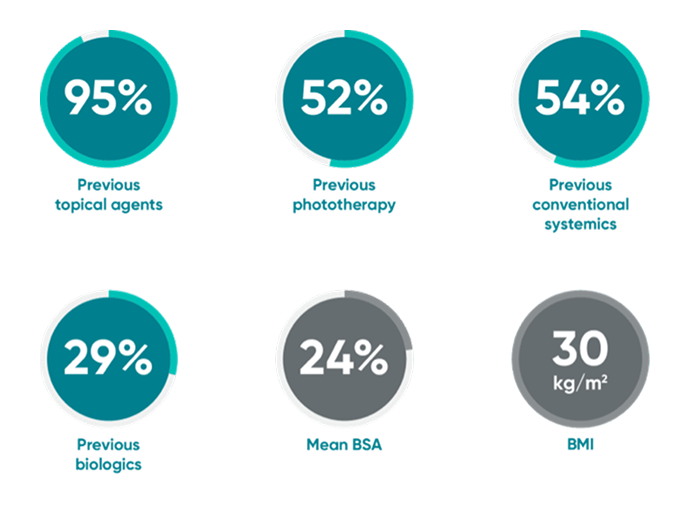
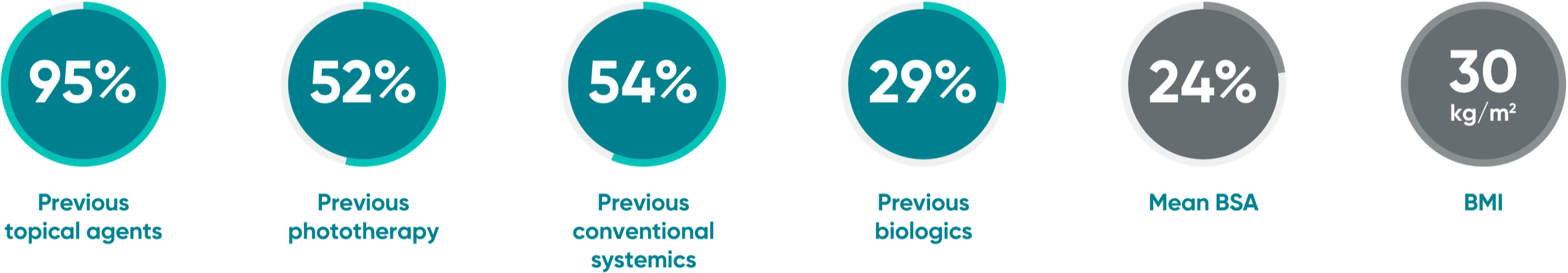
Patient eligibility
- ≥18 years of age
- Moderate to severe plaque psoriasis (IGA score ≥3; PASI score ≥12, BSA involvement ≥10%) for at least 6 months
- Candidates for phototherapy and/or systemic treatment
Overall study population1
ECLIPSE statistical methods
This study utilized a step-down approach to control for multiple testing. The first major secondary endpoint did not achieve statistical significance for superiority; therefore, the remaining P values are nominal and not included in the presentation.
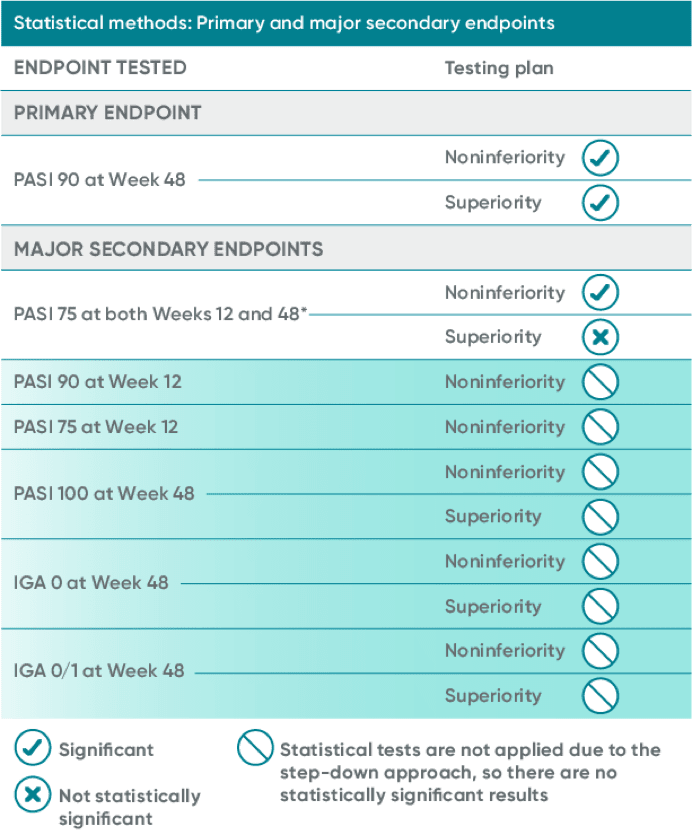

Nonresponder imputation (NRI) methods were used for analysis.
*Superiority for the first major secondary endpoint, PASI 75 at both Week 12 and 48, was not achieved (TREMFYA® 84.6% vs Cosentyx® 80.2%; P=0.062); therefore, the remaining P values are nominal and not included in the presentation.
BMI=body mass index; BSA=body surface area; IGA=Investigator's Global Assessment; PASI=Psoriasis Area and Severity Index.
References: 1. Data on file. Janssen Biotech, Inc. 2. Reich K, Armstrong AW, Langley RG, et al. Guselkumab versus secukinumab for the treatment of moderate-to-severe psoriasis (ECLIPSE): results from a phase 3, randomised controlled trial. Lancet. 2019;394(10201):831-839.
TREMFYA® demonstrated superiority vs Cosentyx® for PASI 90 response at Week 481,2
ECLIPSE: Primary endpoint at Week 48
ECLIPSE: Primary endpoint at Week 48


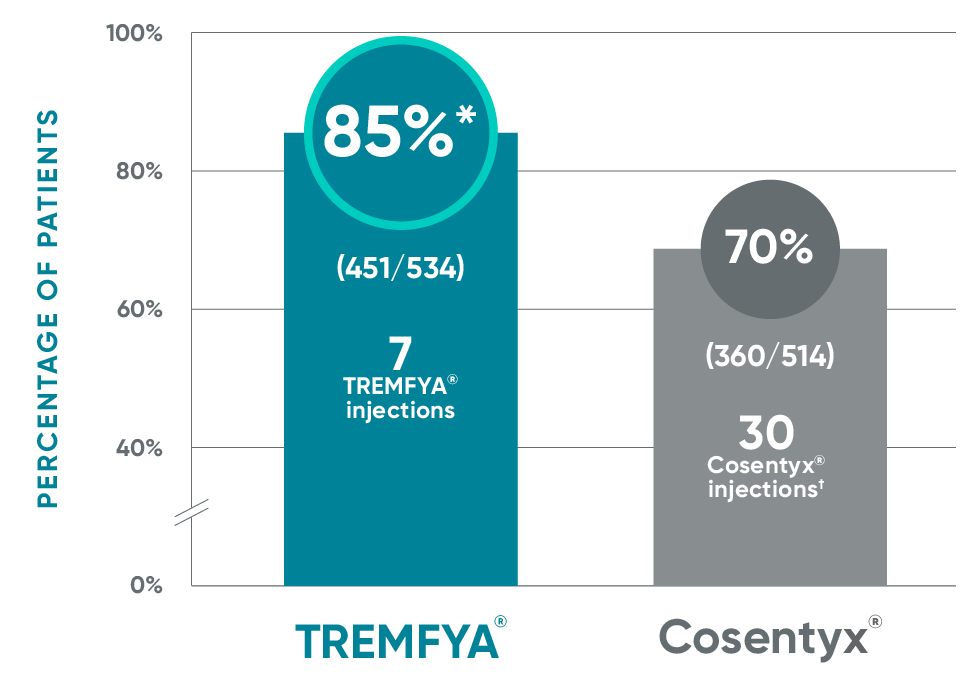
*P<0.001 vs Cosentyx®.
VOYAGE pivotal trials' co-primary endpoints at Week 16 (NRI)2,3:
VOYAGE 1—PASI 90: TREMFYA® 73% (241/329), placebo 3% (5/174) (P<0.001). IGA 0/1: TREMFYA® 85% (280/329), placebo 7% (12/174) (P<0.001). VOYAGE 2—PASI 90: TREMFYA® 70% (347/496), placebo 2% (6/248) (P<0.001). IGA 0/1: TREMFYA® 84% (417/496), placebo 8% (21/248) (P<0.001).
PASI 75 and IGA 0/1 at Week 12 (secondary endpoints at Week 12)
- PASI 75: TREMFYA® 89% (477/534), Cosentyx® 92% (471/514)
- IGA 0/1: TREMFYA® 86% (457/534), Cosentyx® 86% (444/514)
Due to the results of the step-down approach to control for multiple testing, nominal P values for PASI 75 at Week 12 are not presented and efficacy comparisons cannot be made.
IGA 0/1 at Week 12 was a prespecified exploratory endpoint that was not adjusted for multiplicity; P value was considered nominal.
Results based on ECLIPSE, a single study of TREMFYA® vs Cosentyx®.
Nonresponder imputation (NRI) methods were used for analysis.
There were no new safety findings observed for either TREMFYA® or Cosentyx® in this study.
†As of May 2023, Cosentyx® is available in a 150 mg/mL and a 300 mg/3 mL single-dose injection.
IGA=Investigator’s Global Assessment, IGA score of cleared (0) or minimal (1) using a 5-point scale of overall disease severity; PASI=Psoriasis Area and Severity Index.
References: 1. Reich K, Armstrong AW, Langley RG, et al. Guselkumab versus secukinumab for the treatment of moderate-to-severe psoriasis (ECLIPSE): results from a phase 3, randomised controlled trial. Lancet. 2019;394(10201):831-839. 2. Data on file. Janssen Biotech, Inc. 3. TREMFYA® (guselkumab) [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc.
TREMFYA®: IGA 0* at Week 481,2
Due to the results of the step-down approach to control for multiple testing, nominal P values for IGA 0 at Week 48 are not presented and efficacy comparisons cannot be made.
ECLIPSE: Major secondary endpoint at Week 48 (NRI)




At Week 48, 50% (259/514) of patients receiving Cosentyx® achieved IGA 0.
VOYAGE pivotal trials' co-primary endpoints at Week 16 (NRI)2,3:
VOYAGE 1—PASI 90: TREMFYA® 73% (241/329), placebo 3% (5/174) (P<0.001). IGA 0/1: TREMFYA® 85% (280/329), placebo 7% (12/174) (P<0.001). VOYAGE 2—PASI 90: TREMFYA® 70% (347/496), placebo 2% (6/248) (P<0.001). IGA 0/1: TREMFYA® 84% (417/496), placebo 8% (21/248) (P<0.001).
Results based on ECLIPSE: a single study of TREMFYA® vs Cosentyx®.
*IGA 0=proportion of patients who achieved an IGA score of cleared (0) using a 5-point scale where psoriatic lesions are graded by the investigator for induration, erythema, and scaling on a scale of 0 to 4: cleared, except for discoloration (0), minimal (1), mild (2), moderate (3), or severe (4).
References: 1. Reich K, Armstrong AW, Langley RG, et al. Guselkumab versus secukinumab for the treatment of moderate-to-severe psoriasis (ECLIPSE): results from a phase 3, randomised controlled trial. Lancet. 2019;394(10201):831-839. 2. Data on file. Janssen Biotech, Inc. 3. TREMFYA® (guselkumab) [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc.
TREMFYA®: PASI 100*
at Week 481,2
Due to the results of the step-down approach to control for multiple testing, nominal P values for PASI 100 at Week 48 are not presented and efficacy comparisons cannot be made.
ECLIPSE: Major secondary endpoint at Week 48




At Week 48, 48% (249/514) of patients receiving Cosentyx® (secukinumab) achieved PASI 100.
VOYAGE pivotal trials' co-primary endpoints at Week 16 (NRI)2,3:
VOYAGE 1—PASI 90: TREMFYA® 73% (241/329), placebo 3% (5/174) (P<0.001). IGA 0/1: TREMFYA® 85% (280/329), placebo 7% (12/174) (P<0.001). VOYAGE 2—PASI 90: TREMFYA® 70% (347/496), placebo 2% (6/248) (P<0.001). IGA 0/1: TREMFYA® 84% (417/496), placebo 8% (21/248) (P<0.001).
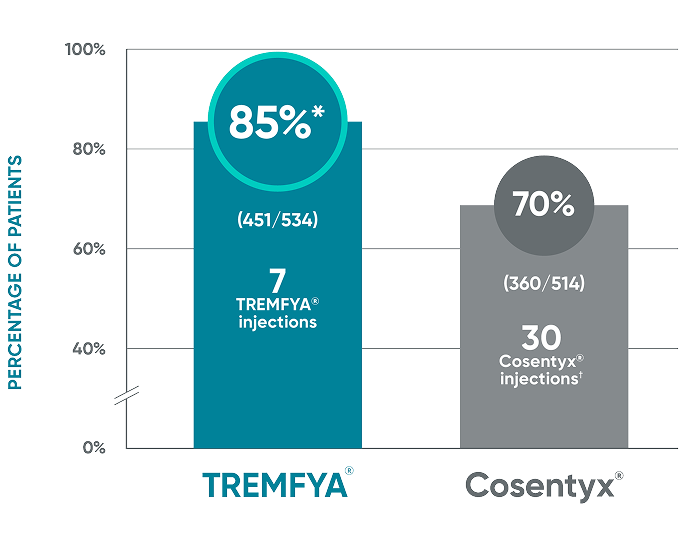
Secondary endpoint at Week 48
- IGA 0/1: TREMFYA® 85% (454/534), Cosentyx® 75% (385/514)1,2
Nonresponder imputation (NRI) methods were used for analysis.
Results based on ECLIPSE: a single study of TREMFYA® vs Cosentyx®.
*PASI 100=proportion of patients who achieved 100% or more reduction (or improvement) in PASI score from baseline.
References: 1. Reich K, Armstrong AW, Langley RG, et al. Guselkumab versus secukinumab for the treatment of moderate-to-severe psoriasis (ECLIPSE): results from a phase 3, randomised controlled trial. Lancet. 2019;394(10201):831-839. 2. Data on file. Janssen Biotech, Inc. 3. TREMFYA® (guselkumab) [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc.
PASI 90 response with TREMFYA® at Week 48
by baseline body weight quartile
ECLIPSE: Post hoc analysis of body weight—PASI 90 at Week 481


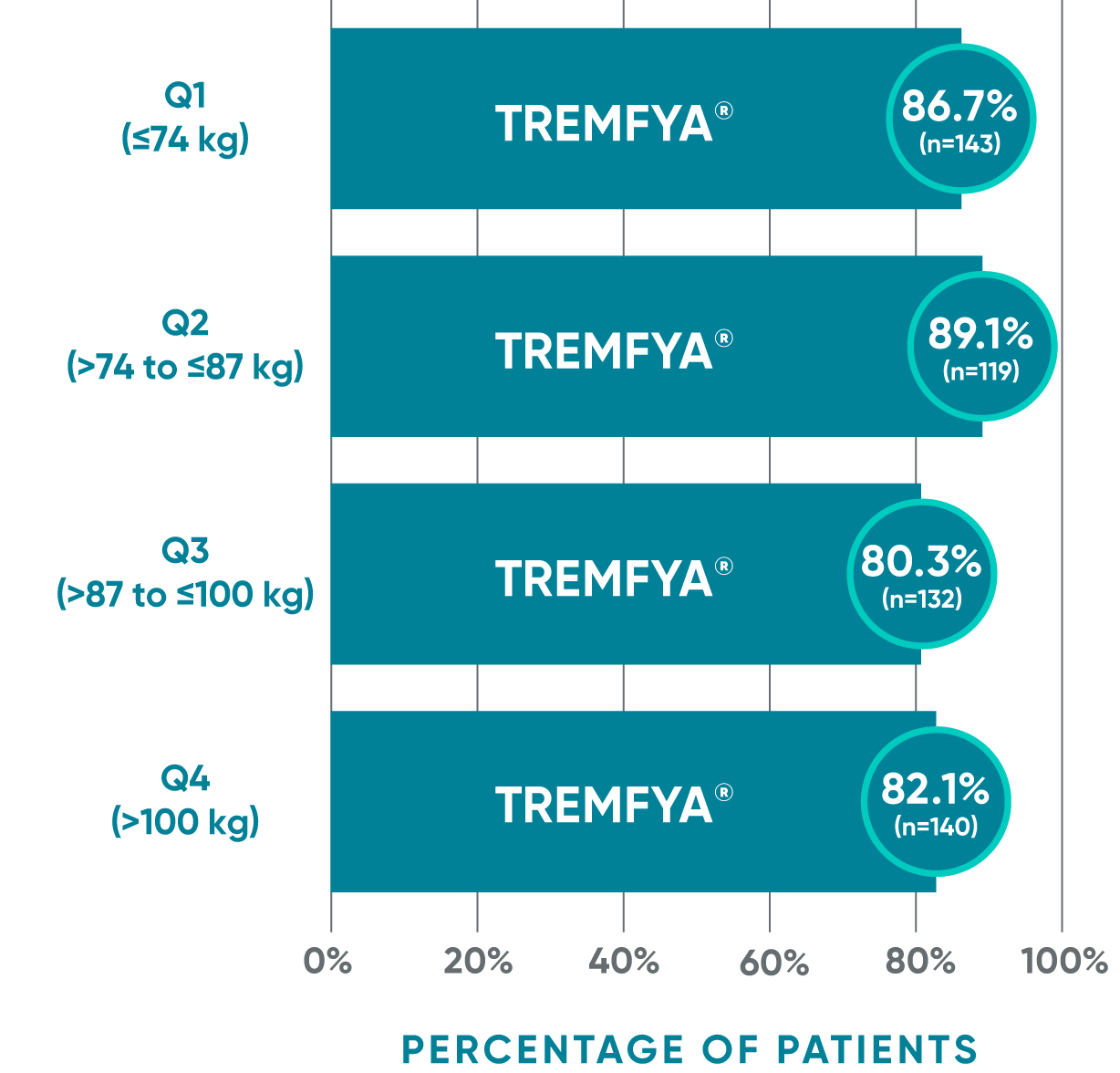

VOYAGE pivotal trials' co-primary endpoints at Week 16 (NRI)2,3:
VOYAGE 1—PASI 90: TREMFYA® 73% (241/329), placebo 3% (5/174) (P<0.001). IGA 0/1: TREMFYA® 85% (280/329), placebo 7% (12/174) (P<0.001). VOYAGE 2—PASI 90: TREMFYA® 70% (347/496), placebo 2% (6/248) (P<0.001). IGA 0/1: TREMFYA® 84% (417/496), placebo 8% (21/248) (P<0.001).
Nonresponder imputation (NRI) methods were used.
This is a post hoc analysis; statistical significance has not been established.
The quartile cutoffs are based on the overall population (not by treatment group).
References: 1. Reich K, Armstrong AW, Langley RG, et al. Guselkumab versus secukinumab for the treatment of moderate-to-severe psoriasis (ECLIPSE): results from a phase 3, randomised controlled trial. Lancet. 2019;394(10201):831-839. 2. TREMFYA® (guselkumab) [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc. 3. Data on file. Janssen Biotech, Inc.
PASI 90 response with TREMFYA® at Week 48 by
baseline BMI category1
ECLIPSE: Prespecified subgroup analysis of BMI category—PASI 90 at Week 48



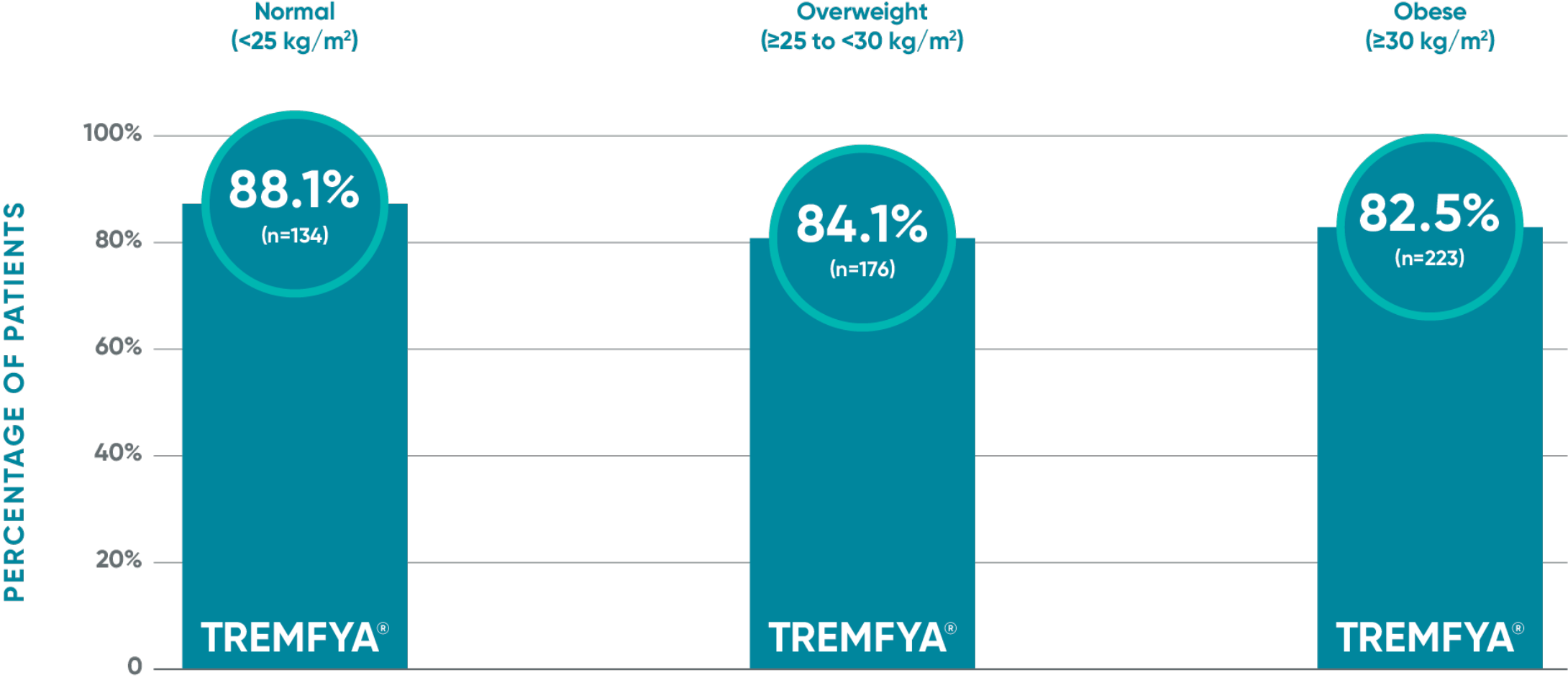
VOYAGE pivotal trials' co-primary endpoints at Week 16 (NRI)2,3:
VOYAGE 1—PASI 90: TREMFYA® 73% (241/329), placebo 3% (5/174) (P<0.001). IGA 0/1: TREMFYA® 85% (280/329), placebo 7% (12/174) (P<0.001). VOYAGE 2—PASI 90: TREMFYA® 70% (347/496), placebo 2% (6/248) (P<0.001). IGA 0/1: TREMFYA® 84% (417/496), placebo 8% (21/248) (P<0.001).
Nonresponder imputation (NRI) methods were used.
This is a post hoc analysis; statistical significance has not been established.
References: 1. Reich K, Armstrong AW, Langley RG, et al. Guselkumab versus secukinumab for the treatment of moderate-to-severe psoriasis (ECLIPSE): results from a phase 3, randomised controlled trial. Lancet. 2019;394(10201):831-839. 2. TREMFYA® (guselkumab) [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc. 3. Data on file. Janssen Biotech, Inc.
IN ADULT PATIENTS WITH MODERATE TO SEVERE PLAQUE PsO
Explore hundreds of real patient
photos with our innovative
Clearance Photo Library
® vs STELARA® (ustekinumab)
IN PATIENTS WITH AN INADEQUATE RESPONSE* TO STELARA®
TREMFYA® demonstrated significant skin clearance1
NAVIGATE: Major secondary endpoint at Week 28 (12 weeks after randomization)†



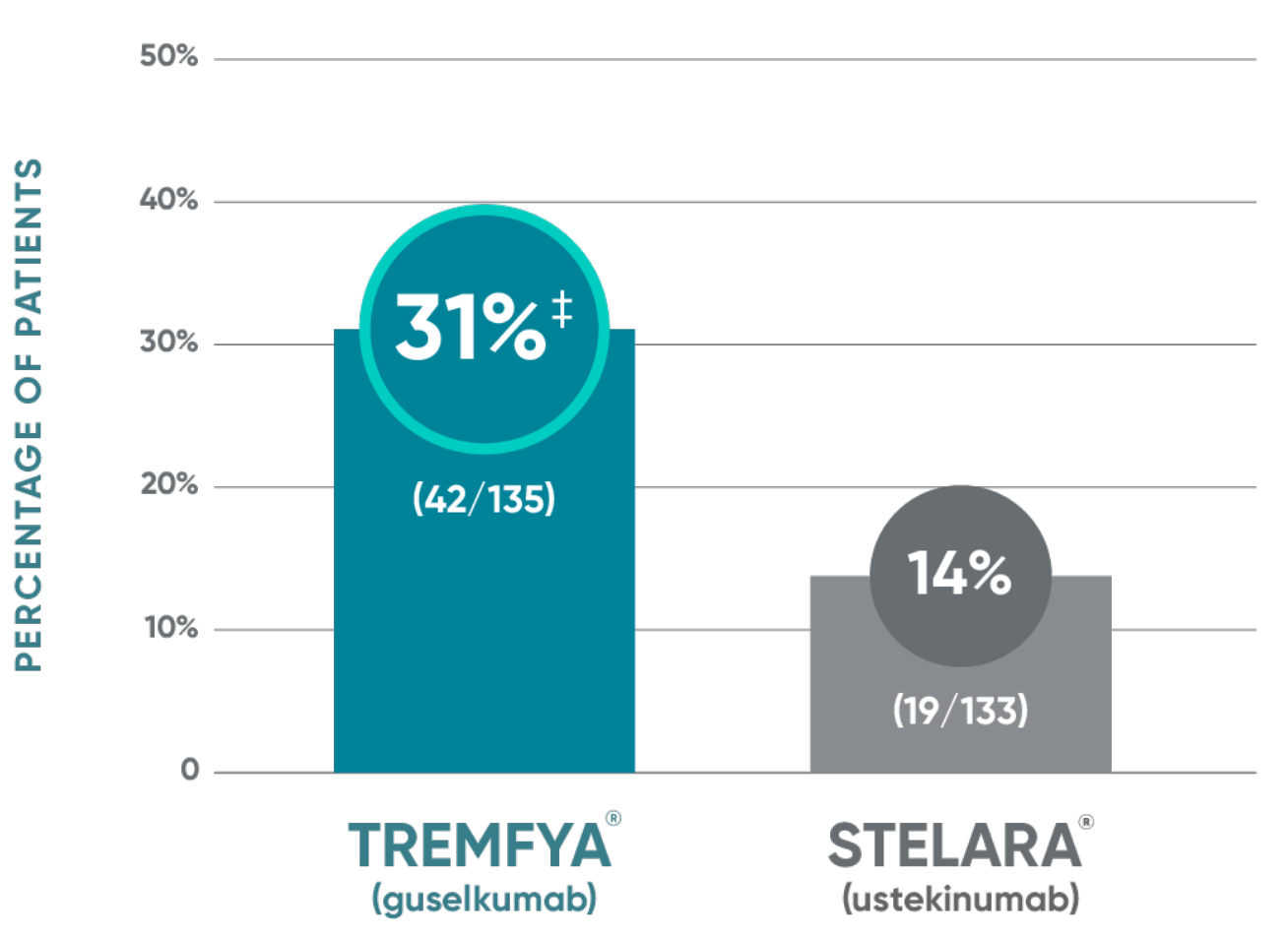
VOYAGE co-primary endpoints at Week 16 (NRI)2,3:
VOYAGE 1—PASI 90: TREMFYA® 73% (241/329), placebo 3% (5/174) (P<0.001). IGA 0/1: TREMFYA® 85% (280/329), placebo 7% (12/174) (P<0.001). VOYAGE 2—PASI 90: TREMFYA® 70% (347/496), placebo 2% (6/248) (P<0.001). IGA 0/1: TREMFYA® 84% (417/496), placebo 8% (21/248) (P<0.001).
*IGA ≥2 at Week 16 after initial treatment with US-licensed STELARA® (dosed 45 mg or 90 mg according to the patient's baseline weight at Week 0 or Week 4).
†More than twice as many patients treated with TREMFYA® achieved IGA 0/1 (with a ≥2-grade improvement) at Week 28.
‡P<0.001 vs STELARA®.
View STELARA® Indication, Important Safety Information, and full Prescribing Information.
References: 1. Langley RG, Tsai, TF, Flavin S, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial. Br J Dermatol. 2018;178(1):114-123. 2. TREMFYA® (guselkumab) [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc. 3. Data on file. Janssen Biotech, Inc.
IN PATIENTS WITH AN INADEQUATE RESPONSE* TO STELARA®
PASI 90 response rate from Week 16 through Week 521
NAVIGATE: Prespecified exploratory endpoint from Week 16 to Week 52†


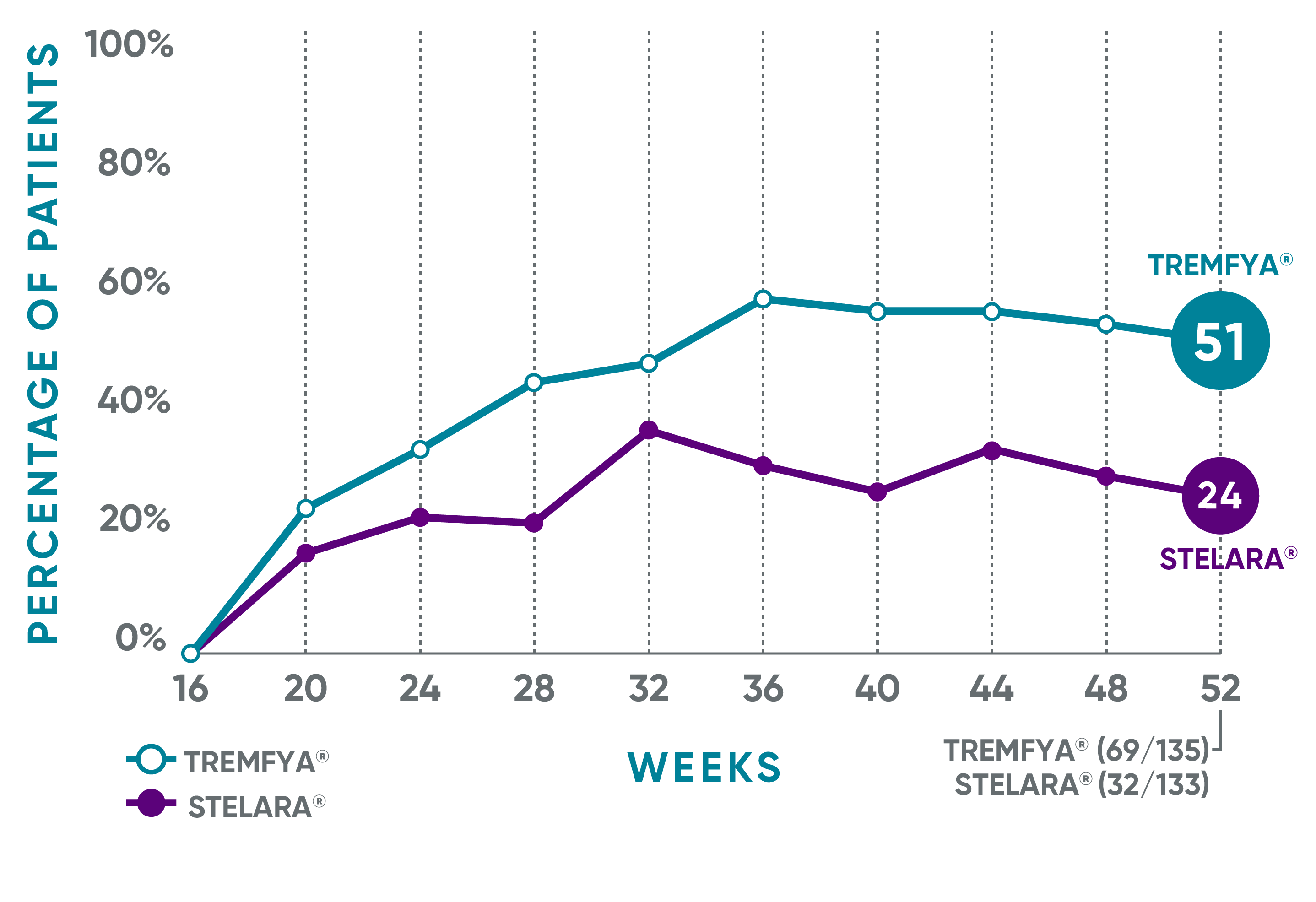
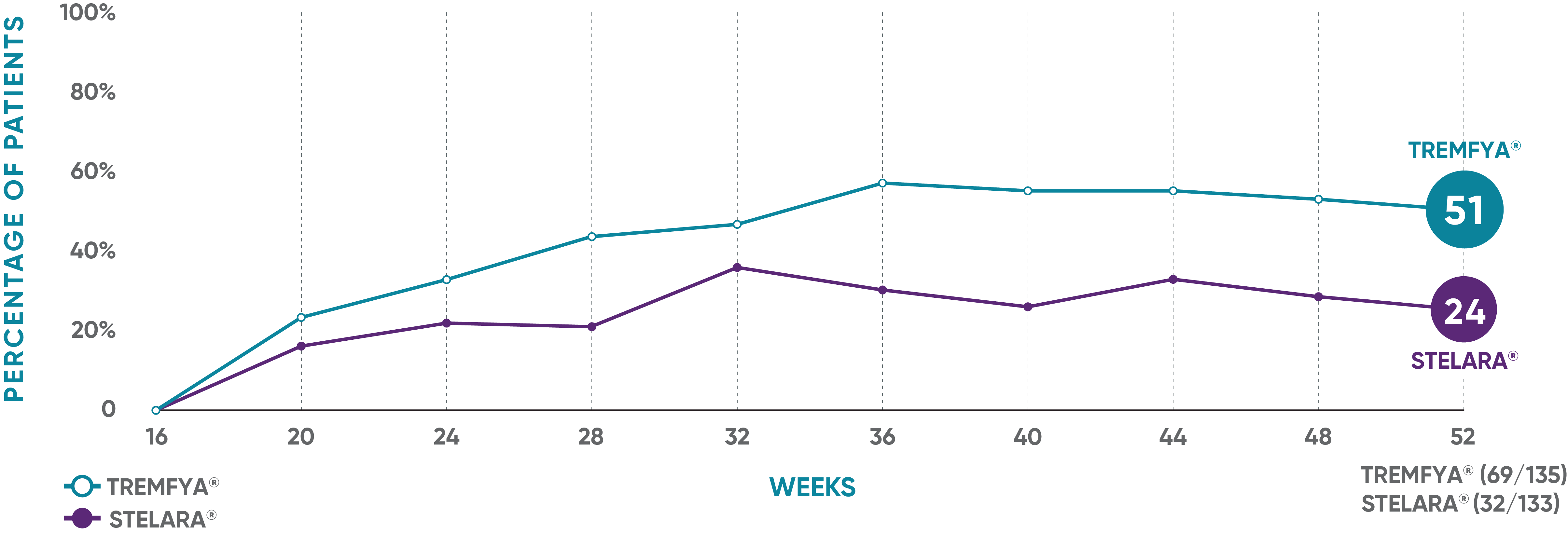
The same patients may not have responded at each time point.
†The proportion of patients who achieved PASI 90 from Week 16 through Week 52 was a prespecified exploratory endpoint that was not adjusted for multiplicity; P values were considered nominal.
VOYAGE pivotal trials’ co-primary endpoints at Week 16 (NRI)2,3:
VOYAGE 1—PASI 90: TREMFYA® 73% (241/329), placebo 3% (5/174) (P<0.001). IGA 0/1: TREMFYA® 85% (280/329), placebo 7% (12/174) (P<0.001). VOYAGE 2—PASI 90: TREMFYA® 70% (347/496), placebo 2% (6/248) (P<0.001). IGA 0/1: TREMFYA® 84% (417/496), placebo 8% (21/248) (P<0.001).
*IGA ≥2 at Week 16 after initial treatment with US-licensed STELARA® (dosed 45 mg or 90 mg according to the patient's baseline weight at Week 0 or Week 4).
PASI=Psoriasis Area and Severity Index.
View STELARA® Indication, Important Safety Information, and full Prescribing Information.
View STELARA® Indication, Important Safety Information, and full Prescribing Information.
References: 1. Langley RG, Tsai, TF, Flavin S, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial. Br J Dermatol. 2018;178(1):114-123. 2. TREMFYA® (guselkumab) [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc. 3. Data on file. Janssen Biotech, Inc.
IN ADULT PATIENTS WITH MODERATE TO SEVERE PLAQUE PsO


